INTRODUCTION
METHODS
The Cancer Genome Atlas (TCGA) analysis
Patients
Immunohistochemistry (IHC)
Cells and treatment
Cell viability assay
Cell cycle detection
Apoptosis detection
RT-qPCR
Table 1
Primers used in the real-time-quantitative polymerase chain reaction assay

Western blot
RESULTS
TP53AIP1 is down-regulated in patients with breast cancer
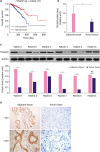 | Figure 1TP53AIP1 is down-regulated in patients with breast cancer. (A) Data from The Cancer Genome Atlas database were collected, and the data showed that breast cancer patients with low level of TP53AIP1 had lower survival rates. (B) Quantitative real-time polymerase chain reaction assay verified down-regulated overall level of TP53AIP1 mRNA in breast cancer patients. (C) Western blot showed down-regulated protein levels of TP53AIP1 in most breast cancer patients, and the representative ones are displayed. (D) Immunohistochemistry assay showed negative protein expression of TP53AIP1 in invasive breast cancer tissues and positive brown TP53AIP1 protein expression in adjacent tissues under 100- and 200-fold magnifications.TP53AIP1 = tumor protein p53-regulated apoptosis-inducing protein 1; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; mRNA = messenger RNA; NS = not significant.
*p < 0.05 and †p < 0.01 vs. adjacent tissue.
|
Overexpression of TP53AIP1 inhibits viability of breast cancer cells
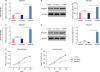 | Figure 2Overexpression of TP53AIP1 inhibits viability of breast cancer cells. RT-qPCR (A) and Western blot (B) were used to show increased mRNA and protein levels of TP53AIP1 in TP53AIP1 (MDA-MB-415) group. RT-qPCR (C) and western blot (D) were used to show increased mRNA and protein levels of TP53AIP1 in TP53AIP1 (MDA-MB-468) group. Cell Counting Kit-8 assay was used to show inhibited cell viabilities in TP53AIP1 (MDA-MB-415) group (E) and TP53AIP1 (MDA-MB-468) group (F).TP53AIP1 = tumor protein p53-regulated apoptosis-inducing protein 1; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; mRNA = messenger RNA; RT-qPCR = real-time-quantitative polymerase chain reaction; NS = not significant; OD = optical density; NC = negative control.
*p < 0.01 vs. NC-vector group.
|
Overexpression of TP53AIP1 promotes cell cycle arrest and apoptosis of breast cancer cells
 | Figure 3Overexpression of TP53AIP1 promotes cell cycle arrest and apoptosis of breast cancer cells. Cell cycle arrest (A) and apoptosis rates (B) were found to be promoted in TP53AIP1 (MDA-MB-415) group. Cell cycle arrest (C) and apoptosis rates (D) were found promoted in TP53AIP1 (MDA-MB-415) group and (MDA-MB-468) group. The rates were determined using flow cytometry.TP53AIP1 = tumor protein p53-regulated apoptosis-inducing protein 1; NC = negative control; FL2 = fidgetin-like 2; NS = not significant.
*p < 0.01 vs. NC-vector group.
|
Overexpression of TP53AIP1 promotes cell cycle arrest and apoptosis of breast cancer cells by regulating cell cycle and apoptosis related factors
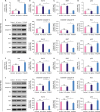 | Figure 4Overexpression of TP53AIP1 promotes cell cycle arrest and apoptosis of breast cancer cells by regulating cell cycle and apoptosis related factors. The mRNA levels of Bax and p53 increased, and Bcl-2 and Ki67 levels decreased in TP53AIP1 (MDA-MB-415) (A-D) and TP53AIP1 (MDA-MB-468) (F-I) groups. The protein levels of cleaved-caspase-3, cleaved-caspase-9, Bax and p53 increased and Bcl-2 and Ki67 levels decreased in TP53AIP1 (MDA-MB-415) (E) and TP53AIP1 (MDA-MB-468) (J) groups.TP53AIP1 = tumor protein p53-regulated apoptosis-inducing protein 1; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; Bcl-2 = B cell lymphoma/leukemia-2; Bax = Bcl-2-associated X protein; mRNA = messenger RNA; NC = negative control; NS = not significant.
*p < 0.05 and †p < 0.01 vs. NC-vector group.
|
Overexpression of TP53AIP1 promotes cell cycle arrest and apoptosis of breast cancer cells via inhibiting the PI3K/Akt pathway
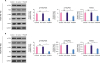 | Figure 5Overexpression of TP53AIP1 promotes cell cycle arrest and apoptosis of breast cancer cells via inhibiting the PI3K/Akt pathway. The percentage of p-PI3K/PI3K, p-Akt/Akt, and Mdm2 levels were suppressed in TP53AIP1 (MDA-MB-415) (A) and TP53AIP1 (MDA-MB-468) (B) groups.TP53AIP1 = tumor protein p53-regulated apoptosis-inducing protein 1; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; NC = negative control; NS = not significant; PI3K = phosphoinositide 3-kinases; Akt = protein kinase B; p-Akt = phosphorylated-Akt; p-PI3K = phosphorylated-PI3K; Mdm2 = mouse double minute 2 homolog.
*p < 0.01 vs. NC-vector group.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download