MATERIALS AND METHODS
The study was approved by the independent ethics committee of Medical University of Gdańsk (NKBBN/344/2018). The database comprised 291 consecutive patients who underwent gastric resection for histologically confirmed adenocarcinoma in two surgical oncology departments in the Pomeranian Region between 2006 and 2013. Mortality data were collected from the polish governmental institution — Ministry of the Digital Development — on November 11, 2017. Information about the date of death in three cases was not available. Meanwhile, the data of 288 patients were complete.
The standard procedure for gastric cancer in both centers was total gastrectomy with appropriate lymphadenectomy. The extent of gastric resection and lymph node dissection was based on disease stage and the judgement of individual surgeon. Resection was routinely followed by Roux-en-Y reconstruction. All procedures were performed via laparotomy. Both centers routinely applied screening for malnutrition using the Nutrition Risk Screening (NRS) 2002 scale. In case of a score ≥3, nutritional support was provided preoperatively. Neoadjuvant chemotherapy was administered in 8.0% of patients. Moreover, total gastrectomy was performed in 245 (85.4%) patients, other organ resection in 10 (3.5%) patients, and subtotal gastric resection in 42 (14.6%) patients. Moreover, 236 (81.9%) procedures had curative intent and 52 (18.1%) had palliative intent. In 214 cases, the most common extent of lymphadenectomy was D1. However, D0 (n=8), D1+ (n=28), and D2 (n=38) procedures were also performed. The average number of resected lymph nodes was 20.1 (median: 19, range: 0–78).
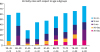
All patients were divided into nine subgroups according to age during surgery. Each group represented 5 years of age, and the groups comprised less than 10 patients (
Fig. 1). For statistical analysis, we stratified patients into four age groups: 29–50 years (group I), 51–65 years (group II), 66–75 years (group III), and 76–92 years (group IV). Statistical calculations focused on the differences in survival and pathoclinical characteristics between groups I and II and between groups II and IV. In addition, to identify statistically significant differences in mortality pattern in group I, we compared the overall survival and middle-term survival rates between this group and the subgroup of patients aged between 51 and 55 years.
Fig. 1
Mortality rates according to age subgroups.
The primary outcomes of this study were early- and middle-term results: 30-day and 3-, 6-, 12-, and 36-month mortality rates.
To validate if the deaths of older patients were correlated to surgery or were just natural consequences of advanced age, we compared the overall survival of patients who lived at least 12 months after gastric resection.
The pathoclinical variables among the groups were as follows: infiltration of the serosa (pT4), nodal involvement (pN+), number of metastatic lymph nodes, total number of resected lymph nodes, extent of lymphadenectomy (D0–D1+ vs. D2–D2+), histological Lauren type of the tumor, tumor location in the stomach (cardia vs. others), presence of distant metastases (M1), stage of the disease according to pTNM (I–II vs. III–IV), neoadjuvant chemotherapy, and postoperative morbidity.
Postoperative morbidity was defined as any serious adverse events according to the Clavien-Dindo classification of surgical complications (grade ≥3) [
9].
The seventh edition of the American Joint Committee on Cancer Staging Manual was used to determine the stage of the disease [
10].
Statistical analysis
Statistical analysis was performed using the data analysis software system Dell Statistica version 13 (Dell Software, Round Rock, TX, USA). Survival was calculated using the Kaplan-Meier method, followed by the log-rank test, to assess the differences between the groups. Comparison of mortality rates was performed using the χ2 test. The pathoclinical variables in the four groups of patients were compared using the χ2 test and Mann-Whitney U test accordingly. A P-value <0.05 was considered statistically significant.
RESULTS
Early- and middle-term mortality rates in the four groups of patients are shown in
Table 1. The Kaplan-Meier curves for overall survival are presented in
Fig. 2. Statistical analysis revealed significant difference between all the groups (P<0.001) and between groups II and IV (P<0.001). Comparison between groups I and II did not show a significant difference (P=0.49). The results of the statistical comparison of mortality rates are presented in
Table 2.
Table 1
Mortality rates in the four groups of patients

|
Group |
No. |
30-day mortality (%) |
3-month mortality (%) |
6-month mortality (%) |
12-month mortality (%) |
36-month mortality (%) |
|
I |
28 |
0 |
3.6 |
14.3 |
25.0 |
60.7 |
|
II |
119 |
0.8 |
5.0 |
8.4 |
16.0 |
41.2 |
|
III |
83 |
7.2 |
12.0 |
16.9 |
28.9 |
59.0 |
|
IV |
58 |
12.1 |
17.2 |
29.3 |
41.4 |
69.0 |
Fig. 2
Overall survival in the four groups of patients in terms of age (P<0.001).

Table 2
Results of the statistical comparison of mortality rates

|
Mortality rate |
P-value |
|
Group I vs. Group II |
Group II vs. Group III |
Group III vs. Group IV |
Group II vs. Group IV |
|
30-day |
0.63 |
0.01 |
0.32 |
<0.001 |
|
3-mo |
0.74 |
0.06 |
0.37 |
0.008 |
|
6-mo |
0.34 |
0.06 |
0.07 |
<0.001 |
|
12-mo |
0.25 |
0.02 |
0.09 |
<0.001 |
|
36-mo |
0.06 |
0.009 |
0.11 |
<0.001 |
In the subgroup of patients aged between 51 and 55 years (n=31), the 30-day, 90-day, 6-month, 12-month, and 36-month mortality rates were 0%, 3.2%, 6.5%, 12.9%, and 29.0% respectively. A significant difference was observed between group I and the 51–55-year-old subgroup in terms of 36-month survival rate (P=0.01). The overall survival (log-rank test) was not statistically different (P=0.3). The 5- and 3-year Kaplan-Meier curves for this comparison are presented in
Fig. 3. Statistically significant difference was observed in terms of 3-year survival, but not for the 5-year survival.
Fig. 3
(A) Comparison of the 5-year survival of group I and patients aged between 51 and 55 years (P=0.09). (B) Comparison of the 3-year survival of group I and patients aged between 51 and 55 years (P=0.02).

Pathological variables in the groups classified according to age are presented in
Table 3. Statistical analysis showed significant differences in nodal involvement, total number of resected lymph nodes, and rate of D2 lymphadenectomy performed between groups II and IV. No relationships between other variables were observed in the groups of patients.
Table 3
Pathological variables in the groups classified according to age

|
Variables |
Group I |
P-value (Group I vs. Group II) |
Group II |
P-value (Group II vs. Group IV) |
Group IV |
|
Infiltration of the serosa (pT4; %) |
28.6 |
0.32 |
38.7 |
0.15 |
27.6 |
|
Nodal involvement (pN1-pN3; %) |
71.4 |
0.79 |
68.9 |
0.01 |
50.0 |
|
Mean/median metastatic nodes |
6.8/2.5 |
0.94 |
6.4/3 |
0.009 |
3.8/1 |
|
Mean (median) total number of resected lymph nodes |
21.8 (21.5) |
0.59 |
21.1 (19) |
0.02 |
17.2 (14.5) |
|
Extend of lymphadenectomy, ≥D2 (%) |
21.4 |
0.64 |
17.6 |
0.02 |
5.1 |
|
Lauren diffused and mixed type (%) |
42.9 |
0.23 |
55.5 |
0.12 |
43.1 |
|
Cardia involvement (%) |
67.9 |
0.51 |
74.0 |
0.83 |
72.4 |
|
Distant metastases (M1; %) |
10.7 |
0.97 |
10.9 |
0.82 |
12.1 |
|
pTNM III–IV (%) |
53.6 |
0.95 |
52.9 |
0.22 |
43.1 |
|
Morbidity rate (%) |
25.0 |
0.38 |
16.7 |
0.01 |
35.6 |
|
Neoadjuvant chemotherapy (%) |
15.0 |
0.47 |
9.5 |
0.3 |
4.4 |
The overall morbidity rate was 22.3%. To identify the impact of postoperative complications on early mortality in older patients, we identified the number of deaths associated with postoperative adverse events in each time period. For this purpose, we combined groups III and IV. The results are presented in
Table 4. The data of 12 patients who died between 2 and 6 months after surgery despite an uneventful postoperative course are presented in
Table 5. The mean length of the postoperative stay of these patients was 9.2 (range: 5–14) days, and only 3 of 12 (25%) patients were diagnosed with cancer recurrence.
Table 4
Impact of postoperative morbidity on mortality periods in 141 patients aged over 65 years

|
Mortality period |
No. of patients (%) |
|
Mortality rate |
Morbidity rate |
|
0–30 day |
13/141 (9.2) |
13/13 (100) |
|
31 day–6 mo |
18/141 (12.8) |
6/18 (33.3) |
|
7–12 mo |
17/141 (12.1) |
4/17 (23.5) |
|
1-yr survival |
93/141 (66.0) |
15/93 (16.1) |
Table 5
Clinicopathologic characteristics of patients who did not present with any complications and who died between 1 and 6 months after surgery

|
Patient |
Sex/age |
pTNM |
Procedure |
Radical/palliative intent |
Day of discharge (since surgery) |
Survival (mo) |
Cancer relapse (yes/no) |
|
1 |
M/66 |
pT4aN3bM0 |
Gastrectomy D1+ |
Curative |
8 |
5 |
Yes |
|
2 |
M/69 |
pT3N0M0 |
Gastrectomy D1 |
Curative |
8 |
5 |
No |
|
3 |
F/70 |
pT4aN3aM0 |
Gastrectomy D1 |
Curative |
8 |
2 |
No |
|
4 |
M/70 |
pT3N3aM0 |
Gastrectomy D2 |
Curative |
12 |
5 |
Yes |
|
5 |
M/71 |
pT2N1M0 |
Gastrectomy D2 |
Curative |
14 |
2 |
No |
|
6 |
F/71 |
pT3N2M0 |
Subtotal resection D1 |
Palliative |
7 |
2 |
No |
|
7 |
F/71 |
pT4aNxM0 |
Subtotal resection D0 |
Palliative |
5 |
4 |
Yes |
|
8 |
F/77 |
pT2N2M0 |
Gastrectomy D1 |
Curative |
9 |
3 |
No |
|
9 |
M/79 |
pT3N3bM0 |
Gastrectomy D1 |
Palliative |
10 |
2 |
No |
|
10 |
M/80 |
pT4aN0M0 |
Gastrectomy D1 |
Curative |
10 |
1 |
No |
|
11 |
M/81 |
pT3N2M0 |
Gastrectomy D1 |
Curative |
11 |
4 |
No |
|
12 |
F/84 |
pT4aN3bM0 |
Gastrectomy D2 |
Curative |
8 |
3 |
No |
The overall survival of patients who lived at least 12 months after gastric resection was compared between groups II and IV, and the data are presented in
Fig. 4. However, the difference was not statistically significant (P=0.16).
Fig. 4
Comparison of the overall survival of patients who lived at least 12 months after gastric resection in groups II and IV (P=0.16).

DISCUSSION
Numerous studies that evaluate the outcome of gastrectomy for cancer in older patients have been conducted [
45678111213]. In most studies, early results have been measured by postoperative morbidity and mortality and long-term results by overall 5-year survival and 5-year disease-specific survival [
246781112]. Although the results are inconsistent due to the variations in methodology, definition of elderly, stratification of patients into two or more age groups, and different outcome measurements, most reports have shown lower overall survival in older patients. However, the disease-specific survival of young and elderly individuals was similar; thus, older patients could safely undergo major surgery [
2461112].
Based on the results of the in-depth analysis of the literature and a closer look on our own patients' follow-up data, we decided to assess this issue and presented the outcome of patients who underwent gastrectomy for cancer according to age groups with emphasis on middle-term mortality patterns.
After dividing the patients into nine subgroups according to age (
Fig. 1), we stratified them into four groups. The patients aged under 50 years had different mortality pattern compared with the middle-aged patients. Therefore, a separate group for the youngest individuals (group I) was established. The stratification revealed that the 6-month mortality rates were higher than 29% in group IV and approximately 17% in group III. We combined groups III and IV and identified the association between postoperative complications and middle-term mortality. As shown in
Table 4, two-thirds of patients who died between 30 days and 6 months after surgery did not present with any serious postoperative adverse events. Moreover, the 1- and 3-year mortality rates remained significantly higher in group IV than in group II (both P<0.001), with a tendency to equalize only after 5 years since the surgical procedure (P=0.012).
This tendency may be at least partly due to less invasive tumor growth and metastatic potential in older patients [
11]. We found significantly less nodal involvement in group IV (
Table 4). In the literature, the observations regarding the differences in tumor behavior between young and old individuals are controversial [
456781114]. Some studies have shown similar clinicopathologic characteristics between young and old individuals [
47], whereas other studies have presented various disparities in pathological features among older adults [
56811]. Japanese patients aged over 80 years had more commonly well-differentiated tumors located in the lower third of the stomach [
11]. Meanwhile, Chinese individuals aged over 65 years presented with tumor in the upper third of the stomach, which is a more common tumor site, and more advanced stage of the disease [
5]. Furthermore, Korean octogenarians had more advanced stage of the disease [
8], whereas Italian patients aged over 70 years presented with tumor in the intestine, which is more common [
6].
A discrepancy was also observed in terms of age between Eastern and Western populations, and this should be considered in the process of patient selection for surgery after obtaining data from the literature. The cut-off age of patients with the highest postoperative risk may be different among countries. For example, Japan is known for their greatest longevity among other countries, with a population ratio of 22.7% for individuals aged ≥65 years, in 2009 [
11]. Meanwhile, those of Poland and the USA are 13.5% [
15] and 12.75%, respectively [
16]. Takeshita et al. [
11] have concluded that the radical resection of gastric tumors should have been considered as the first-line treatment for patients aged between 80 and 84 years. However, gastrectomy was not highly considered in patients aged over 85 years.
In our opinion, another issue is that the outcomes of the oldest group of patients should not be compared to the outcomes of the rest of individuals who underwent surgery. The youngest patients usually present with a more aggressive type of the disease with rapid progression and dissemination, and their mortality pattern is different from that observed in other groups of patients [
171819]. To identify the statistically significant differences in mortality among the youngest patients, we compared them to the subgroup of patients (aged 51–55 years) with the best prognosis, as observed in
Fig. 1. As shown in
Figs. 2 and
3, the youngest patients were more likely to die between 5 and 30 months after surgery, and deaths were not observed after 40 months since surgery. These results might be attributed to the significant differences in 3-year survival, not the 5-year survival. Although no statistically significant differences were observed due to the relatively small number of youngest patients, notably, the 6-month mortality rate was higher in group I than in group II despite the excellent outcomes after 30 days and 3 months. The 5-year survival after curative gastrectomy in young patients was found to be similar or even better than that in older patients [
202122]; nevertheless, based on the in-depth analysis of the studies, we concluded that these results are not conflicting with our findings.
Isobe et al. [
23] have shown significantly lower 5-year survival rate in patients younger than 40 years compared with all the patients who underwent gastric resection. However, when comparing only curative cases, which were defined as the presence of clear margins, without or minimal serosal invasion, N2 nodal involvement or less, absence of tumor invasion in the last lymph node resected barrier, and absence of distant metastasis, the survival rates in young patients and the other group pf patients were similar. Another issue is comparing the results of the youngest group of patients with the rest of individuals who underwent surgery. Saito et al. [
18] have classified 1,731 patients into three groups: aged ≤40 years, between 40 and 70 years, and >70 years. In the literature, this was the only study that identified middle-aged patients with significantly better 5-year survival than that of either young or elderly individuals. However, no differences were observed in the prognosis between patients aged ≤40 years and all other patients aged >40 years. Therefore, based on the study by Saito et al. [
18], we strongly recommend classifying patients into more than two groups when studying the prognosis of both elderly and young individuals.
Authors have shown similar disease-specific survival between older and younger patients after excluding patients who died from causes other than cancer recurrence [
61112]. Italian authors have shown significantly worse overall 3- and 5-year survival in patients aged over 70 years who were undergoing curative gastric resection, and they have concluded that age is not a limiting factor in patient selection for surgery and that the differences in survival rates were attributed to the shorter life expectancy of elderly individuals [
6]. Kunisaki et al. [
12] have stated that the reason for the poor prognosis among patients aged over 75 years was the high rate of mortality caused by other cancers or comorbidities. Based on the analysis of the Kaplan-Meier curves showing overall survival after potentially curative resection in multiple reports, we can estimate the 12-month mortality rate of older patients, which range from 25% to 40% [
5678]. In our opinion, the deaths were correlated to the natural consequences of advanced age, not the past surgery, and such results may not be in accordance with the those of the comparison of the overall survival of patients between groups II and IV who lived at least 12 months after gastric resection (
Fig. 4).
Tran et al. [
2] have assessed the outcomes of gastric cancer resection in patients older than 80 years. The authors have found significantly higher rates of postoperative complications and 30-day and 90-day mortality rates in these patients. Because the 5-year survival rates were similar in octogenarians and younger patients, the authors have concluded that the long-term cancer-specific outcome was similar in both younger and older patients if the surgery had been performed safely [
2]. Although not always literally expressed, this conclusion differs from those of other studies about the outcomes of gastrectomy for cancer in older adults [
468111213]. Because this is quite contrary to the findings of the current study, we decided to present the issue about gastrectomy in elderly individuals in terms of middle-term results.
The process of aging is associated with multiple progressing adverse phenomena. The most substantial phenomena are sarcopenia and frailty [
2425]. Major surgery in older patients, by way of extensive perioperative trauma, leads to decline in physiologic reserves and increase in deficits across multiple organ systems that eventually deteriorates the quality and length of life. Gastrectomy itself involves serious disturbances in dietary intake and absorption leading to the escalation of malnutrition, which then causes frailty and sarcopenia.
Our results showed that long-terms outcomes and 30-day morbidity and mortality in older patients must be assessed to evaluate the efficacy and safety of major surgery for gastric cancer. A high number of patients aged over 75 years die within few months after discharge, even in cases of successful surgical procedure with a completely uneventful postoperative course. Sakurai et al. [
13] have pointed out the problem of declining activity below the preoperative baseline. In the elderly group (>80 years), a higher percentage of patients were admitted to care hospitals, mostly due to requirement of parenteral nutrition and rehabilitation. The surgery itself declines whole body and mind functions, and this does not only increase short-term postoperative morbidity but also lead to the aggravation of symptoms of aging and downgrading of the quality of life of older adults. Considering the oldest patients qualified for major surgery may lead to a higher number of iatrogenic premature deaths, which is not necessarily a cause of postoperative adverse events but a natural consequence of postoperative trauma. All these findings should be considered in the selection of older patients for surgical treatment and must set the expectations for postoperative recovery.
The primary limitation of this study is its retrospective nature and lack of reliable frailty extent evaluation before surgery. We recommend that future studies should assess which factors, except chronological age, may affect 6-month mortality in patients aged over 75 years. Such observations could be used to guide the stratification of older patients for gastric resection or to identify the best supportive care or at least to make more informed decisions. Nevertheless, our study first showed the extremely high mortality rate among older patients in a short period of time after undergoing a successful gastric resection. This problem was overlooked in the literature probably due to the lack of assessment of middle-term results and inclusion of patients aged under 50 years to the control group, when stratifying the patients into two groups only, for the comparison of outcomes between older and younger individuals.
Although advanced age per se does not reflect biological functions and patients aged over 75 years are a heterogenous population [
24], age itself is an important prognostic factor of middle-term survival after gastric resection of tumors. However, geriatric assessment and better knowledge of how to predict the long-term outcome of major surgery for cancer are required to improve the decision-making process in patient selection for gastrectomy for cancer.










 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download