Abstract
Purpose
To evaluate the long-term treatment outcomes of bevacizumab therapy in patients with myopic choroidal neo-vascularization (CNV).
Methods
A retrospective review was performed of medical records regarding 43 eyes with myopic CNV that were treated with intravitreal bevacizumab injection. Visual acuity at diagnosis was compared with that measured at the final follow-up; the incidence and timing of re-activation were evaluated. In addition, factors associated with final follow-up were analyzed.
Results
Mean patient age was 39.3 ± 12.9 years and mean spherical equivalent (SE) was −11.9 ± 4.4 diopters. Patients were fol-lowed-up at a mean of 42.1 ± 17.0 months. Re-activation of the lesion was noted in 17 eyes (39.5%). The mean time to first re-activation was 19.5 ± 15.4 months from the time that resolution of subretinal fluid/retinal fluid was confirmed after initial treatment. The mean visual acuity (the logarithm of the minimal angle of resolution) was 0.40 ± 0.25 at diagnosis and 0.26 ± 0.31 at the final follow-up. Visual acuity at the final follow-up was significantly improved when compared with the baseline value (p = 0.005). Patient age (p < 0.001), SE (p = 0.003), and visual acuity at diagnosis (p < 0.001) were significantly associated with visual acuity at the final follow-up.
References
1. Wong TY, Ferreira A, Hughes R, et al. Epidemiology and disease burden of pathologic myopia and myopic choroidal abdominal: an evidence-based systematic review. Am J Ophthalmol. 2014; 157:9–25.e12.
2. Ohno-Matsui K, Yoshida T. Myopic choroidal neovascularization: natural course and treatment. Curr Opin Ophthalmol. 2004; 15:197–202.

3. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neo-vascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.

4. Rich RM, Rosenfeld PJ, Puliafito CA, et al. abdominal safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina. 2006; 26:495–511.
5. Silva RM, Ruiz-Moreno JM, Nascimento J, et al. abdominal abdominal and safety of intravitreal ranibizumab for myopic choroidal neovascularization. Retina. 2008; 28:1117–23.
6. Yamamoto I, Rogers AH, Reichel E, et al. Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularisation secondary to pathological myopia. Br J Ophthalmol. 2007; 91:157–60.

7. Willis J, Morse L, Vitale S, et al. Treatment patterns for myopic choroidal neovascularization in the United States: analysis of the IRIS registry. Ophthalmology. 2017; 124:935–43.
8. Chhablani J, Paulose RM, Lasave AF, et al. Intravitreal abdominal monotherapy in myopic choroidal neovascularisation: 5-year outcomes for the PAN-American Collaborative Retina Study Group. Br J Ophthalmol. 2018; 102:455–9.
9. Tan NW, Ohno-Matsui K, Koh HJ, et al. abdominal outcomes of ranibizumab treatment of myopic choroidal neovascularization in east-asian patients from the radiance study. Retina. 2018; 38:2228–38.
10. Kung YH, Wu TT, Huang YH. One-year outcome of two different initial dosing regimens of intravitreal ranibizumab for myopic abdominal neovascularization. Acta Ophthalmol. 2014; 92:e615–20.
11. Lim EH, Jang YS, Lew YJ, Yoo SJ. Comparison of two doses of IVB and prognostic factor on myopic CNV: 1-year outcome. J Korean Ophthalmol Soc. 2012; 53:1807–13.
12. Cha DM, Kim TW, Heo JW, et al. Comparison of 1-year abdominal effect of ranibizumab and bevacizumab for myopic abdominal neovascularization: a retrospective, multicenter, comparative study. BMC Ophthalmol. 2014; 14:69.

13. Kim KH, Jung JH, Lee JE, Oum BS. Clinical effect of intravitreal bevacizumab injection in myopic choroidal neovascularization. J Korean Ophthalmol Soc. 2010; 51:359–65.

14. Oh HN, Lee JE, Kim HW, Yun IH. Predictive factors for visual abdominal after treatment for myopic choroidal neovascularization. J Korean Ophthalmol Soc. 2013; 54:610–7.
15. Seo YS, Chang MH. abdominal therapeutic effect of intravitreal bevacizumab (Avastin) on myopic choroidal neovascularization. J Korean Ophthalmol Soc. 2011; 52:34–40.
16. Jung SI, Han JS, Kwon JW, et al. Analysis of myopic progression in childhood using the Korea National Health and Nutrition Examination Survey. J Korean Ophthalmol Soc. 2016; 57:1430–4.

17. Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of abdominal and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016; 123:1036–42.
18. Song MH, Kim JY, Roh YJ. abdominal efficacy of intravitreal ranibizumab for myopic choroidal neovascularization. J Korean Ophthalmol Soc. 2009; 50:1027–34.
19. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007; 143:566–83.

20. Ohno-Matsui K, Yoshida T, Futagami S, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal abdominal in pathological myopia. Br J Ophthalmol. 2003; 87:570–3.
21. Yang HS, Kim JG, Kim JT, Joe SG. Prognostic factors of eyes with naïve subfoveal myopic choroidal neovascularization after abdominal bevacizumab. Am J Ophthalmol. 2013; 156:1201–10.e2.
22. Yoshida T, Ohno-Matsui K, Ohtake Y, et al. abdominal visual abdominal of choroidal neovascularization in high myopia: a abdominal between age groups. Ophthalmology. 2002; 109:712–9.
23. Nishida Y, Fujiwara T, Imamura Y, et al. Choroidal thickness and visual acuity in highly myopic eyes. Retina. 2012; 32:1229–36.

24. Moghadas Sharif N, Shoeibi N, Ehsaei A, Atchison D. Structure versus function in high myopia using optical coherence abdominal and automated perimetry. Clin Exp Optom. 2019; 102:335–40.
25. Al-Sheikh M, Phasukkijwatana N, Dolz-Marco R, et al. Quantitative OCT angiography of the retinal microvasculature and the chorioca-pillaris in myopic eyes. Invest Ophthalmol Vis Sci. 2017; 58:2063–9.

26. Miyake M, Yamashiro K, Akagi-Kurashige Y, et al. Vascular abdominal growth factor gene and the response to anti-vascular abdominal growth factor treatment for choroidal neovascularization in high myopia. Ophthalmology. 2014; 121:225–33.
27. Lee SC, Cho KW, Kim NS. Subretinal hemorrhage in high myopia. J Korean Ophthalmol Soc. 1996; 37:596–601.
Figure 1.
Clinical course of a 52 year-old patient who was diagnosed with myopic choroidal neovascularization (CNV) in the left eye. At diagnosis, myopic fundus (A) and active leakage from CNV was noted on fluorescein angiography (B), and subretinal fluid with hyper-reflective material was noted on optical coherence tomography (C). The patient was treated with 3 monthly intravitreal bevacizumab injections. One month after the third injection (D), the fluid had completely resolved. At 48 months after the confirmation of the fluid resolution, first re-activation of the lesion was noted accompanied with the development of retinal hemorrhage (E, F). The patient received additional bevacizumab injection and the fluid and hemorrhage had completely resolved one month after the treatment (G).
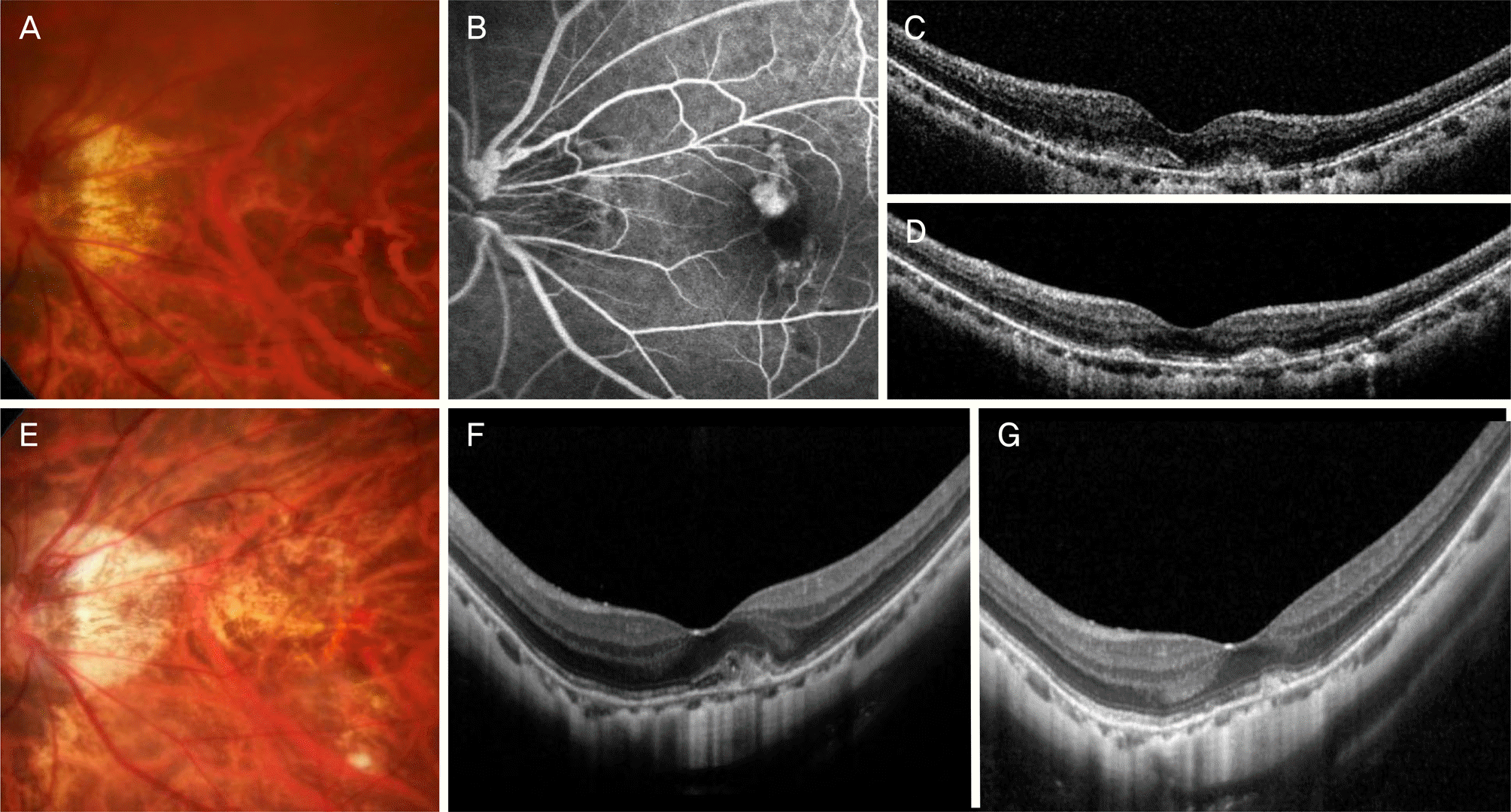
Figure 2.
A Kaplan-Meier curve showing the cumulative re-activation of the myopic choroidal neovascularization after identification of complete resolution of fluid following the initial treatment. Among the 43 eyes, 17 eyes (39.5%) showed re-activation of the lesion during the follow-up period.
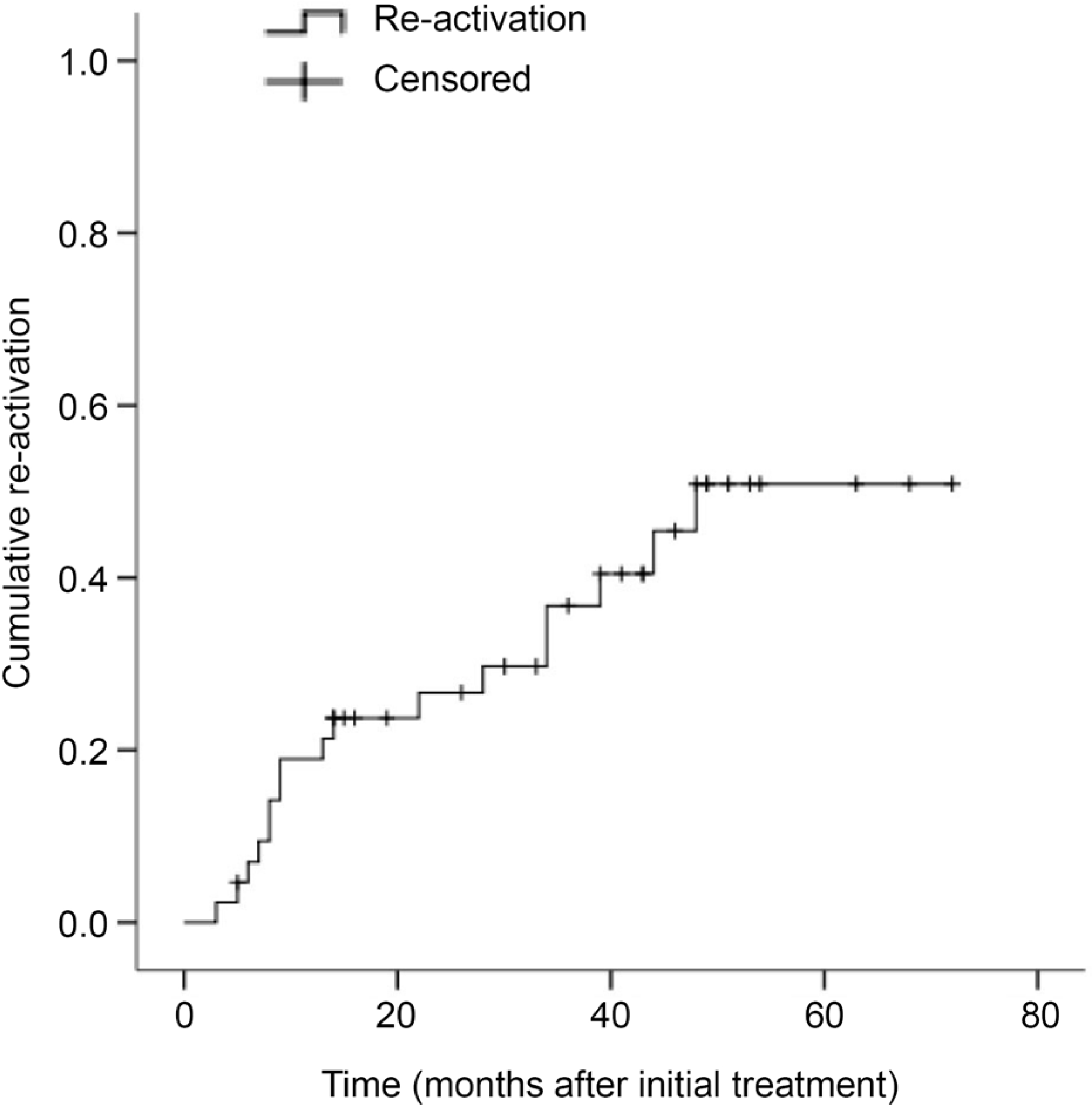
Figure 3.
Changes in BCVA in patients with myopic choroidal neovascularization who were treated with intravitreal bevacizumab. Statistical analysis was performed using repeated measures analysis of variances with a Bonferroni's correction. ‘Final visit’ showed mean 42.1 ± 17.0 months. BCVA = best-corrected visual acuity; logMAR = the logarithm of minimal angle of resolution.
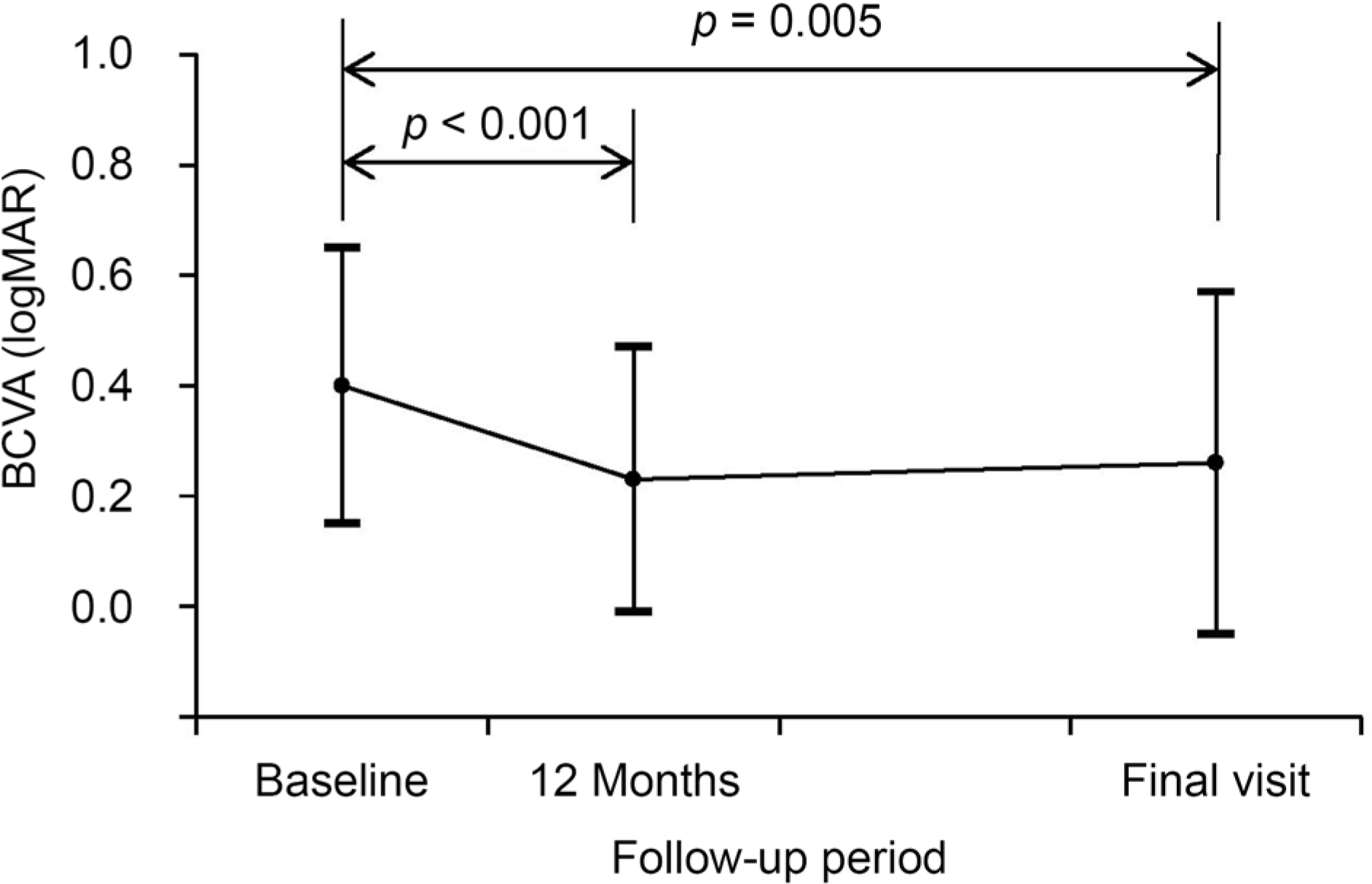
Table 1.
Baseline characteristics of 43 patients (43 eyes) who were diagnosed with myopic choroidal neovascularization
Table 2.
Associations between characteristics and best-cor-rected visual acuity at the final visit (n = 43)
| Characteristic | p-value* |
|---|---|
| Age (years) | <0.001 |
| Spherical equivalents | 0.003 |
| Best-corrected visual acuity at diagnosis | <0.001 |
| Central retinal thickness (μ m) | 0.974 |
| Number of bevacizumab injection as an initial therapy | 0.406 |
| Total number of bevacizumab injection | 0.113 |
| Follow-up period | 0.387 |
Table 3.
Associations between characteristics and best-cor-rected visual acuity at the final visit (n = 43)
| Characteristic | p-value (confidence interval)* |
|---|---|
| Age (years) | 0.001 (0.004–0.016) |
| Spherical equivalents | 0.043 (−0.040 to −0.001) |
| Best-corrected visual acuity at diagnosis | 0.001 (0.273–0.880) |
| Central retinal thickness (μ m) | 0.861 |
| Number of bevacizumab injection as an initial therapy | 0.335 |
| Total number of bevacizumab injection | 0.290 |
| Follow-up period | 0.435 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download