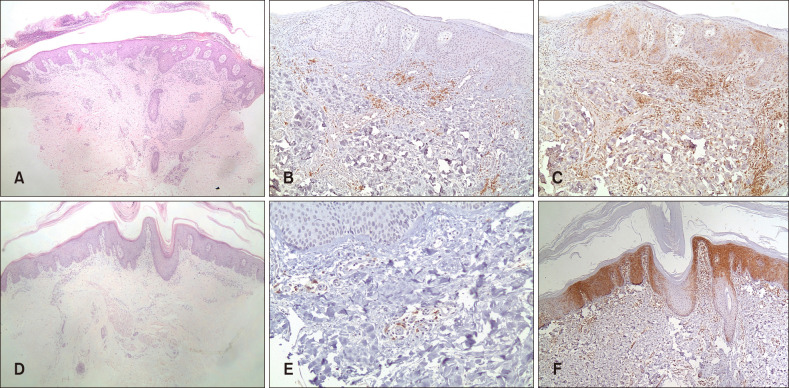
 | Fig. 1(A~C) Psoriasis. (A) Representative case showing classical microscopic features of psoriasis (H&E, ×40). (B) Blood-derived dendritic cell antigen-2 (BDCA-2) immunostaining highlighted plasmacytoid dendritic cells (pDCs) in a superficial perivascular distribution (×100). (C) Patchy myxovirus protein A (MxA) immunostaining of both the epithelium and inflammatory cells (×100). (D~F) PRP. (D) Representative case showing alternating ortho- and parakeratosis, follicular plugging, psoriasiform acanthosis and underlying dermal inflammatory infiltrate (H&E, ×40). (E) BDCA-2 immunostaining highlighted pDCs in a superficial perivascular distribution (×200). (F) Patchy MxA immunostaining of both the epithelium and inflammatory cells (×100). |
Dear Editor:
Plasmacytoid dendritic cells (pDCs) are bone marrow-derived DCs with plasma cell morphology. They are lineage negative and express CD4, CD123, HLA-DR, blood-derived dendritic cell antigen-2 (BDCA-2) and Toll-like receptor (TLR) 7 and TLR9 within endosomal compartments1. When activated, TLRs initiate a cascade of multiple signaling pathways that ultimately lead to production of pro-inflammatory cytokines such as type I interferons (INFs) and, to a lesser extent, IL-6 and TNF-α. This cytokine profile, especially type I IFN, is responsible for pDCs' anti-viral effect as well as their ability to link innate and adaptive immunity1. In addition to their role in cancer immunity and cutaneous viral infections, pDCs have pathogenetically been implicated in several inflammatory skin diseases such as lupus erythematosus (LE), psoriasis, lichen planus, alopecia areata, among others1.
Pytiriasis rubra pilaris (PRP) is a rare idiopathic papulosquamous inflammatory skin disorder, with heterogeneous clinical manifestations, characterized by follicular keratosis, orange-red scaling plaques with “Islands of sparing” and palmoplantar hyperkeratosis2. PRP shares with psoriasis many overlapping features. The presence of a focally thickened stratum granulosum, follicular plugging and foci of acantholysis are helpful tools in favoring PRP2. PRP pathogenesis is still poorly understood. The role of abnormal vitamin A metabolism in the skin with disruption of the retinoid signaling pathways has long been considered as a potential inducer of increased epidermal proliferation2. Genetics also play a role in PRP. An autosomal dominant familial type was recently demonstrated with mutations in CARD14 gene3. This gene is also implicated in familial psoriasis, making familial PRP a possible allelic variant to familial psoriasis3. Rarely, CARD14 genetic mutations have also been demonstrated in sporadic PRP cases3. Given the overlapping clinicopathological and genetic features between PRP and psoriasis and since pDCs have been implicated in psoriasis pathogenesis, we intend to investigate any role of pDCs in PRP, which has not been studied before.
Our institutional review board approved the study (IRB no. DER.OA.12). Twenty one PRP and 18 psoriasis vulgaris cases were retrieved from our database. Only straight-forward cases were included. None of the patients was receiving treatment when the biopsy was performed. Immunohistochemical analysis was performed on sections obtained from formalin-fixed, paraffin-embedded tissue using antibodies to BDCA-2 (mouse immuno-globulin G1, clone 124B3.13; Dendritics, Lyon, France) and myxovirus resistance protein A (MxA, M143; University of Freiburg, Freiburg, Germany, Professor Haller). Anti-BDCA2 antibody is a specific pDC marker1, while anti-MxA antibody assesses type I IFN production by pDCs, since MxA is well established surrogate marker for local type I IFN production1. A semiquantitative scoring system was used to assess pDC recruitment and MxA expression. pDCs content was scored as percentage of the total mononuclear infiltrate: 0 (absent or very rare positive cells), 1 (1%~10% of positive cells), 2 (10%~50% of positive cells), 3 (>50% of positive cells). MxA staining was scored as: 0 (negative), 1 (weak/patchy staining), and 2 (intense/diffuse staining). Normal skin tissue served as negative control (pDC score of 0, MxA score of 0) and discoid LE served as positive control (pDC score of 3, MxA score of 2) (Table 1).
PRP patients (13 men and 8 women; 14 type I and 7 type III) ranged in age from 6 to 69 years (mean of 39). Disease onset prior to biopsy ranged between 1 to 4 weeks. Psoriasis patients (8 men and 10 women) had all psoriasis vulgaris and ranged in age from 10 to 72 years (mean of 44 years). Disease onset ranged between 3 and 9 weeks. Results (Fig. 1) showed the pDCs to be present in 18/21 (86%) PRP cases and 18/18 (100%) psoriasis cases and pDCs were significantly less abundant in PRP than in psoriasis cases. Patchy weak MxA expression was similarly present in both PRP and psoriasis cases.
Our hypothesis in this study concerning pDC role in PRP pathogenesis is based on several observations. First, PRP has been reported in association with different viral infections such as herpes virus infections, hepatitis A and C, and HIV, which is associated with type VI PRP45. It is not surprising that pDCs may be implicated in such immune pathways, since they are known to be key effectors in innate antiviral immunity1. Second, PRP has been associated with several inflammatory disorders such as LE, lichen planus, alopecia areata and vitiligo56, in which evidence suggests significant pDC role in their underlying pathogenesis1. Third, imiquimod, an immunomodulator known to be a potent pDC activator through its effect on TLR-7, has been reported to induce or exacerbate PRP7. Fourth, PRP relation to psoriasis may raise the possibility of similar underlying pathogenesis. In addition to the genetic and clinicopathological overlap between PRP and psoriasis, recent evidence suggests shared pathogenic pathways8. Similar to psoriasis, a recent study identified a role of the IL-23-TH17-axis in PRP8. In the study, upregulated expression of most proinflammatory innate cytokines (TNF, IL-6, IL-12, IL-23, and IL-1β) and adaptive T-cell cytokines such as TH1 and, especially, TH17 (IL-17A, IL-17F, and IL-22) cytokines was demonstrated in lesional PRP skin lesions. Clinical and histopathologic improvement of PRP lesions in one patient treated with ustekinumab (human anti-IL-12/IL-23 antibody) paralleled the expression levels of TH17 cytokines, further supporting the role of IL-23-TH17-axis in PRP. Activated pDC and type I IFNs are now obviously implicated in the initial innate immune events that drive inflammation in psoriasis9. pDCs infiltrate early psoriatic skin lesions in correlation with expression of markers typical of early psoriasis phases9. In psoriatic skin, extracellular self-DNA fragments get coupled to overexpressed endogenous antimicrobial peptides. This breaks innate tolerance via TLR9 activation and leads to pDC activation and IFN production9. In addition, pDC relation to IL-23-TH17-axis, implicated in psoriasis and PRP, has recently been partially clarified. A recent study demonstrated that imiquimod-stimulated pDCs can initiate Th17-deviated inflammation in humans, which partially explains reports of imiquimod inducing or exacerbating psoriasis and PRP10.
In conclusion, we have demonstrated pDC presence as part of the inflammatory infiltrate in most PRP cases suggesting possible role of pDCs in PRP, possibly in the initial steps of disease onset similar to psoriasis.
References
1. Saadeh D, Kurban M, Abbas O. Update on the role of plasmacytoid dendritic cells in inflammatory/autoimmune skin diseases. Exp Dermatol. 2016; 25:415–421. PMID: 26837058.

2. Marrouche N, Kurban M, Kibbi AG, Abbas O. Pityriasis rubra pilaris: clinicopathological study of 32 cases from Lebanon. Int J Dermatol. 2014; 53:434–439. PMID: 24783259.

3. Takeichi T, Sugiura K, Nomura T, Sakamoto T, Ogawa Y, Oiso N, et al. Pityriasis rubra pilaris type V as an autoinflammatory disease by CARD14 mutations. JAMA Dermatol. 2017; 153:66–70. PMID: 27760266.

4. Wang T, Liu J, Liu Y, Zheng H. Pityriasis rubra pilaris (PRP) with preceding Epstein-Barr virus infection: a new type PRP with non-HIV virus infection? Chin Med J (Engl). 2014; 127:391. PMID: 24438637.
5. Cecchi R, Giomi A, Tuci F, Bartoli L, Seghieri G. Pityriasis rubra pilaris, Lichen planus, Alopecia universalis and vitiligo in a patient with chronic viral hepatitis C. Dermatology. 1994; 188:239–240. PMID: 8186518.

6. Lerner MR, Braverman IM. Psoriasis, lupus erythematosus, and Pityriasis rubra pilaris: occurrence in one family. Arch dermatol. 1962; 85:229–231. PMID: 14464250.
7. Atanaskova Mesinkovska N, Dawes D, Sood A, Bergfeld W. Acantholytic Pityriasis rubra pilaris associated with imiquimod 3.75% application. Case Rep Dermatol Med. 2011; 2011:412684. PMID: 23198175.
8. Feldmeyer L, Mylonas A, Demaria O, Mennella A, Yawalkar N, Laffitte E, et al. Interleukin 23-helper T cell 17 axis as a treatment target for Pityriasis rubra pilaris. JAMA Dermatol. 2017; 153:304–308.

9. Lande R, Chamilos G, Ganguly D, Demaria O, Frasca L, Durr S, et al. Cationic antimicrobial peptides in psoriatic skin cooperate to break innate tolerance to self-DNA. Eur J Immunol. 2015; 45:203–213. PMID: 25332209.

10. Garzorz-Stark N, Lauffer F, Krause L, Thomas J, Atenhan A, Franz R, et al. Toll-like receptor 7/8 agonists stimulate plasmacytoid dendritic cells to initiate a TH17-deviated acute contact dermatitis in human subjects. J Allergy Clin Immunol. 2018; 141:1320–1333.e11. PMID: 28935206.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download