INTRODUCTION
Scarring is an inevitable result of the wound healing process
1. Although scars are not representative of malignant lesions, they can have significant physical and emotional impacts on human life
2345. Scarring can occur on any parts of the body, but facial scars can seriously impair a patient's quality of life
5. Therefore, there has been a growing interest in preventing and minimizing facial scars in recent years. Various methods such as the use of silicone agents, intralesional steroid injection, 5-fluorouracil, laser therapy, radiation therapy, cryotherapy, bleomycin, and pressure therapy have been proposed
67. In recent decades, laser therapy has been recognized as one of the most effective treatment methods for scars and many studies have provided evidence for its benefit
68910. Different types of lasers such as pulsed-dye laser (PDL), non-ablative fractional laser (NAFL), and ablative fractional laser (AFL) have been used for different types of scars
1410111213.
Various factors, including patient characteristics and treatment-related factors, such as optimal treatment time and treatment modalities, can influence treatment outcomes. However, standardized guidelines for facial scar treatment have yet to be established. This study was designed to evaluate the factors that may play an important role in scar treatment and offer an optimal guideline for facial scar treatment.
Go to :

RESULTS
Eighty-four scars of 64 patients were analyzed in this study. Most of the patients had a single facial scar (n=48), and 16 patients had two or more scars. Overall, the median NTSE was 4 (range 1~10) sessions. The median interval between treatment sessions was 6 (range 3~10) weeks. The median treatment duration was 18.5 (range 3~65) weeks. The median initial VSS score was 5 (range 1~9), which reduced to 2 (range 0~4) at the treatment endpoint.
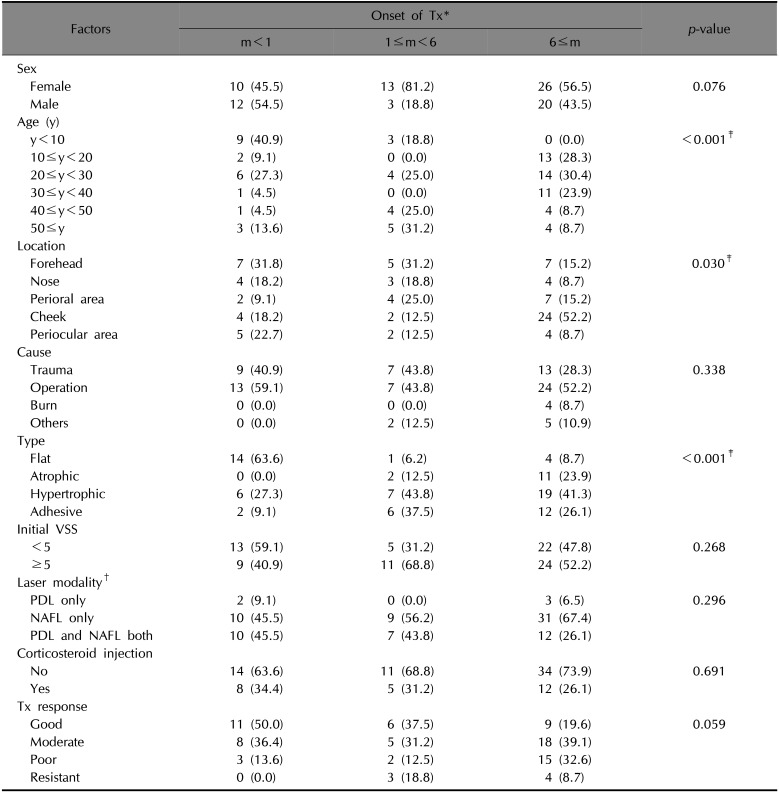
When scars were grouped by OT (m), the m<1, 1≤m<6, and m≥6 groups differed significantly from each other based on several other factors (
Table 1). When the laser treatment started earlier, more scars were observed in the better treatment response group, but differences between the OT groups were not statistically significant (
p=0.0590). Scars of the m<1 group were more likely to be located on the forehead, while scars of the m>6 group were more likely to be located on the cheeks (
p=0.030). Flat scars were the most frequently occurring among the four scar types in the m<1 group (63.6%); however, hypertrophic scars were more frequent in the 1≤m<6 (43.8%) and m≥6 (41.3%) groups (
p<0.001).
Table 1
Different characteristics of facial scars according to treatment onset
|
Factors |
Onset of Tx*
|
p-value |
|
m<1 |
1≤m<6 |
6≤m |
|
Sex |
|
|
|
|
|
Female |
10 (45.5) |
13 (81.2) |
26 (56.5) |
0.076 |
|
Male |
12 (54.5) |
3 (18.8) |
20 (43.5) |
|
|
Age (y) |
|
|
|
|
|
y<10 |
9 (40.9) |
3 (18.8) |
0 (0.0) |
<0.001‡
|
|
10≤y<20 |
2 (9.1) |
0 (0.0) |
13 (28.3) |
|
|
20≤y<30 |
6 (27.3) |
4 (25.0) |
14 (30.4) |
|
|
30≤y<40 |
1 (4.5) |
0 (0.0) |
11 (23.9) |
|
|
40≤y<50 |
1 (4.5) |
4 (25.0) |
4 (8.7) |
|
|
50≤y |
3 (13.6) |
5 (31.2) |
4 (8.7) |
|
|
Location |
|
|
|
|
|
Forehead |
7 (31.8) |
5 (31.2) |
7 (15.2) |
0.030‡
|
|
Nose |
4 (18.2) |
3 (18.8) |
4 (8.7) |
|
|
Perioral area |
2 (9.1) |
4 (25.0) |
7 (15.2) |
|
|
Cheek |
4 (18.2) |
2 (12.5) |
24 (52.2) |
|
|
Periocular area |
5 (22.7) |
2 (12.5) |
4 (8.7) |
|
|
Cause |
|
|
|
|
|
Trauma |
9 (40.9) |
7 (43.8) |
13 (28.3) |
0.338 |
|
Operation |
13 (59.1) |
7 (43.8) |
24 (52.2) |
|
|
Burn |
0 (0.0) |
0 (0.0) |
4 (8.7) |
|
|
Others |
0 (0.0) |
2 (12.5) |
5 (10.9) |
|
|
Type |
|
|
|
|
|
Flat |
14 (63.6) |
1 (6.2) |
4 (8.7) |
<0.001‡
|
|
Atrophic |
0 (0.0) |
2 (12.5) |
11 (23.9) |
|
|
Hypertrophic |
6 (27.3) |
7 (43.8) |
19 (41.3) |
|
|
Adhesive |
2 (9.1) |
6 (37.5) |
12 (26.1) |
|
|
Initial VSS |
|
|
|
|
|
<5 |
13 (59.1) |
5 (31.2) |
22 (47.8) |
0.268 |
|
≥5 |
|
|
|
|
|
Laser modality†
|
9 (40.9) |
11 (68.8) |
24 (52.2) |
|
|
PDL only |
2 (9.1) |
0 (0.0) |
3 (6.5) |
0.296 |
|
NAFL only |
10 (45.5) |
9 (56.2) |
31 (67.4) |
|
|
PDL and NAFL both |
10 (45.5) |
7 (43.8) |
12 (26.1) |
|
|
Corticosteroid injection |
|
|
|
|
|
No |
14 (63.6) |
11 (68.8) |
34 (73.9) |
0.691 |
|
Yes |
8 (34.4) |
5 (31.2) |
12 (26.1) |
|
|
Tx response |
|
|
|
|
|
Good |
11 (50.0) |
6 (37.5) |
9 (19.6) |
0.059 |
|
Moderate |
8 (36.4) |
5 (31.2) |
18 (39.1) |
|
|
Poor |
3 (13.6) |
2 (12.5) |
15 (32.6) |
|
|
Resistant |
0 (0.0) |
3 (18.8) |
4 (8.7) |
|

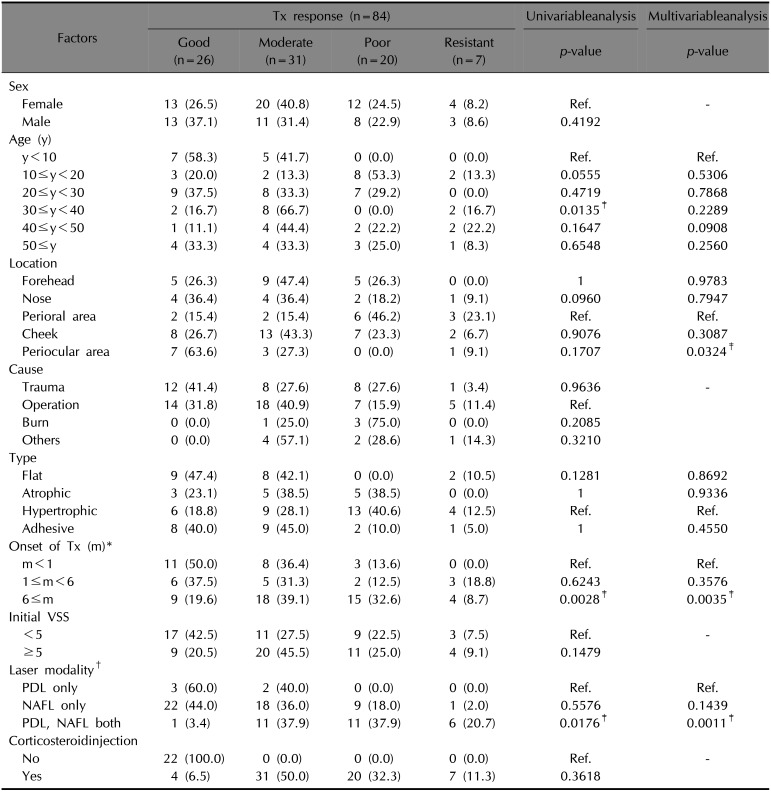
In univariable analyses, age, OT, and laser modalities (LM) were significantly associated with treatment response (
Table 2). Subsequently, a multivariable analysis was performed considering the variables which were statistically significant in the univariable analysis and the potential confounders from the comparison of the OT groups. As a result, only three factors were found to be significantly associated with treatment response; OT, LM, and the location of the scar. Among the OT groups, there was a significant difference in the treatment response of the scar treated earlier within a month and the scar treated after 6 months (
p=0.0035). Considering scar locations, particularly, scars on the perioral area and scars on the periocular area showed a significant difference in treatment response (
p=0.0324). The group of patients who were treated using both PDL and NAFL showed a significantly worse treatment response when compared to the group of patients treated using only PDL (
p=0.0011).
Table 2
Univariable and multivariable analyses of the effects of various factors on treatment response
|
Factors |
Tx response (n=84) |
Univariableanalysis |
Multivariableanalysis |
|
Good (n=26) |
Moderate (n=31) |
Poor (n=20) |
Resistant (n=7) |
p-value |
p-value |
|
Sex |
|
|
|
|
|
|
|
Female |
13 (26.5) |
20 (40.8) |
12 (24.5) |
4 (8.2) |
Ref. |
- |
|
Male |
13 (37.1) |
11 (31.4) |
8 (22.9) |
3 (8.6) |
0.4192 |
|
|
Age (y) |
|
|
|
|
|
|
|
y<10 |
7 (58.3) |
5 (41.7) |
0 (0.0) |
0 (0.0) |
Ref. |
Ref. |
|
10≤y<20 |
3 (20.0) |
2 (13.3) |
8 (53.3) |
2 (13.3) |
0.0555 |
0.5306 |
|
20≤y<30 |
9 (37.5) |
8 (33.3) |
7 (29.2) |
0 0.0 |
0.4719 |
0.7868 |
|
30≤y<40 |
2 (16.7) |
8 (66.7) |
0 (0.0) |
2 (16.7) |
0.0135‡
|
0.2289 |
|
40≤y<50 |
1 (11.1) |
4 (44.4) |
2 (22.2) |
2 (22.2) |
0.1647 |
0.0908 |
|
50≤y |
4 (33.3) |
4 (33.3) |
3 (25.0) |
1 (8.3) |
0.6548 |
0.2560 |
|
Location |
|
|
|
|
|
|
|
Forehead |
5 (26.3) |
9 (47.4) |
5 (26.3) |
0 0.0 |
1 |
0.9783 |
|
Nose |
4 (36.4) |
4 (36.4) |
2 (18.2) |
1 (9.1) |
0.0960 |
0.7947 |
|
Perioral area |
2 (15.4) |
2 (15.4) |
6 (46.2) |
3 (23.1) |
Ref. |
Ref. |
|
Cheek |
8 (26.7) |
13 (43.3) |
7 (23.3) |
2 (6.7) |
0.9076 |
0.3087 |
|
Periocular area |
7 (63.6) |
3 (27.3) |
0 (0.0) |
1 (9.1) |
0.1707 |
0.0324‡
|
|
Cause |
|
|
|
|
|
|
|
Trauma |
12 (41.4) |
8 (27.6) |
8 (27.6) |
1 (3.4) |
0.9636 |
- |
|
Operation |
14 (31.8) |
18 (40.9) |
7 (15.9) |
5 (11.4) |
Ref. |
|
|
Burn |
0 (0.0) |
1 (25.0) |
3 (75.0) |
0 (0.0) |
0.2085 |
|
|
Others |
0 (0.0) |
4 (57.1) |
2 (28.6) |
1 (14.3) |
0.3210 |
|
|
Type |
|
|
|
|
|
|
|
Flat |
9 (47.4) |
8 (42.1) |
0 (0.0) |
2 (10.5) |
0.1281 |
0.8692 |
|
Atrophic |
3 (23.1) |
5 (38.5) |
5 (38.5) |
0 0.0 |
1 |
0.9336 |
|
Hypertrophic |
6 (18.8) |
9 (28.1) |
13 (40.6) |
4 (12.5) |
Ref. |
Ref. |
|
Adhesive |
8 (40.0) |
9 (45.0) |
2 (10.0) |
1 (5.0) |
1 |
0.4550 |
|
Onset of Tx (m)*
|
|
|
|
|
|
|
|
m<1 |
11 (50.0) |
8 (36.4) |
3 (13.6) |
0 0.0 |
Ref. |
Ref. |
|
1≤m<6 |
6 (37.5) |
5 (31.3) |
2 (12.5) |
3 (18.8) |
0.6243 |
0.3576 |
|
6≤m |
9 (19.6) |
18 (39.1) |
15 (32.6) |
4 (8.7) |
0.0028‡
|
0.0035‡
|
|
Initial VSS |
|
|
|
|
|
|
|
<5 |
17 (42.5) |
11 (27.5) |
9 (22.5) |
3 (7.5) |
Ref. |
- |
|
≥5 |
9 (20.5) |
20 (45.5) |
11 (25.0) |
4 (9.1) |
0.1479 |
|
|
Laser modality†
|
|
|
|
|
|
|
|
PDL only |
3 (60.0) |
2 (40.0) |
0 (0.0) |
0 (0.0) |
Ref. |
Ref. |
|
NAFL only |
22 (44.0) |
18 (36.0) |
9 (18.0) |
1 (2.0) |
0.5576 |
0.1439 |
|
PDL, NAFL both |
1 (3.4) |
11 (37.9) |
11 (37.9) |
6 (20.7) |
0.0176‡
|
0.0011‡
|
|
Corticosteroidinjection |
|
|
|
|
|
|
|
No |
22 (100.0) |
0 (0.0) |
|
0 (0.0) |
Ref. |
- |
|
Yes |
4 (6.5) |
31 (50.0) |
20 (32.3) |
7 (11.3) |
0.3618 |
|

Go to :

DISCUSSION
This study was designed to identify the valuable factors for predicting treatment response of laser treatment for facial scars. With the help of biostatistics specialists, we analyzed possible influencing factors based on their treatment response, for which we determined values by the NTSE. NTSE was defined as the number of treatments needed to achieve a VSS decrease of ≥50%, compared with the initial score. Therefore, the smaller NTSE values indicated the better treatment response and the larger NTSE values vice versa.
Among many treatment modalities, lasers have been successfully used for scar treatment, and among them, 585-nm or 595-nm PDL and NAFL have been consistently reported to be effective and safe
17111718. In this study, scars were treated with one of these two lasers based on a treatment algorithm. Corticosteroid injection (CSI) is the most popularly used treatment modality for scarring
1920. However, for facial scars, cosmetic concerns are a priority and, therefore, possible adverse effects such as steroid-induced atrophy or CSI-related telangiectasia are not often acceptable to patients. For this reason, we limited the use of CSI to the lowest concentration possible and used it only for scars with high pliability scores.
In the present study, scars showed a tendency of having better treatment response, when laser treatment was initiated earlier following injury. The optimal timing for initiating laser scar treatment has not yet been determined. Traditionally, laser treatments are started after the wound had matured, which meant several months after trauma or surgery
1115. Some researchers have reported that laser treatment could be effectively started 2~3 months after scar stabilization and when erythema had decreased at the surgical site
21. However, earlier interventions have been emphasized in recent years to prevent hypertrophic scar formation
15. Studies have shown that earlier interventions using PDL could be started without waiting for the wound to mature
18. It has also been reported that early treatment with fractional lasers, such as AFL or NAFL, is beneficial
1516222324.
In general, the progression of a wound healing process comprises of the following phases; the inflammation phase for a few days, the proliferation phase for weeks, and the maturation phase for several months or years. Previous studies have shown that hypertrophic scars generally begin to develop 6 to 8 weeks after wound healing, grow rapidly for 3~6 months, and then gradually regress after 6 months
2526. Lasers generate heat to cause inflammation and increase the permeability of blood vessels, matrix metalloproteinase production, and degradation of collagen fibers
1718. Moreover, targeted blood-vessel destruction induces hypoxia in the tissue, thereby reducing fibroblast proliferation and interfering with collagen deposition
1718. Thus, early implementation of laser treatment can shorten the acute inflammation stage, accelerate scar maturation, and prevent excessive scar formation
17. Consistently, the results of the current study suggested that early active intervention of scars may reduce the number of treatments and show better treatment response rather than the conventional wait-and-see or delayed scar treatment until the natural course of scar maturation.
Interestingly, our results showed that the location of the scar was also significantly related to treatment outcome and especially scars on the perioral area had worse treatment response than scars on other areas. Particular areas of the body are more likely to develop hypertrophic scars, especially the front chest, shoulder, and the lower abdomen
727. This might be due to different degrees of mechanical tension at each region
728. The perioral area is also an example of a highly movable area that is associated with larger mechanical tension due to the attachment of masticatory or facial muscles. Therefore, scars occurring on the perioral area are expected to respond poorly to the treatment.
The superiority of certain laser modality over others in scar treatment has not been reported until now
91617. However, the present study showed the statistically significant association of LM with treatment response in the multivariable analysis, which could be due to the nature of our treatment algorithm in which only one type of laser can be used in a single treatment session. To be precise, if both PDL and NAFL had been used on a scar, it was evident that the scar must have been treated at least twice. However, in the PDL only group or the NAFL only group, patients treated with only one session of each laser treatment is included. Therefore, among the three groups of LM, in the group using both PDL and NAFL, NTSE could inevitably be predicted to be higher than the groups using only one modality among PDL and NAFL.
This study has a few limitations. First, because this study was the retrospective analysis of patients, comparative analysis with the untreated control groups was not carried out. Second, incomplete clinical data and follow-up loss limited the number of scars that we could include in the analyses. Third, the exclusion criteria eliminating the patients who had stopped receiving treatments before reaching the endpoint of treatment might obtain biased results with better treatment response. Fourth, the use of different detailed treatment methods, depending on individual scar characteristics, was a serious analysis concern. However, all the scars were treated by one laser specialist following the same algorithm, and LM was controlled in our multivariable analysis. Lastly, the study population was limited to Asian patients with Fitzpatrick skin type III or IV.
Despite these limitations, the discovery that OT is associated with treatment response is of major clinical significance. In the daily practice, patients with facial scars often prefer to get treated as soon as possible and to the maximum extent possible and do not wish to wait for the natural course of scar maturation. Our results provide indirect evidence to the argument that early active laser intervention for scars yields better and faster outcomes compared to the conventional wait-and-see scar treatment that recommends delayed treatment until scar maturation.
Although we investigated the effects of several factors on the scar-treatment response, other factors may also affect the outcomes of scar treatment. In addition to the factors analyzed in the present study, anemia, ischemia, hormones, stress, smoking, alcohol consumption, obesity and body mass index, medications, and underlying conditions (immunocompromised, diabetes, malnutrition, etc.) are known to influence wound healing
182930. Therefore, in future studies, the above-mentioned factors should be considered.
In conclusion, the most important factors associated with the treatment response in facial scars were the location of the scar and the timing of the start of treatment. The latter seemed to be the only modifiable factor for patients to achieve better treatment response among the factors we analyzed. Therefore, physicians should advise patients with a facial scar that early active intervention may help them in reducing the number of treatment sessions required, save cost and time, and achieve more desirable clinical outcomes. In addition, our results revealed that scars on different locations may show a different response to the treatment. Especially, patients with scars on the perioral area might require more treatment sessions. Such information can be used to predict treatment response and to tailor the treatment plan, depending on the characteristics of the scar. To ensure the generalizability of these results, further research is required involving larger populations at multiple centers over a longer period of time.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download