INTRODUCTION
Atopic dermatitis (AD) is an inflammatory skin disorder characterized by relapsing eczema with intractable itching
1. Besides well-known allergic conditions, including asthma, allergic rhinitis, and allergic conjunctivitis, many other comorbidities have recently been associated with AD
2. Ocular disorders in patients with AD have been widely acknowledged and frequently reported worldwide. Specifically, cataract, retinal detachment, blepharitis, glaucoma, keratoconjunctivitis, and keratoconus are associated with AD
3456. However, the ocular disorders of AD patients are not of primary concern to most dermatologists, particularly if patients do not complain of associated symptoms. If left untreated, cataract and glaucoma in AD patients may progress to the advanced stages, resulting in irreversible vision loss.
To the best of our knowledge, only a few studies have used a large-sample, population-based study design to investigate possible relationships between AD and ocular disease. The aim of the present study was therefore to use a population-based design to determine whether cataract, glaucoma, and dry eye disease are associated with AD in an adult Korean population.
Go to :

MATERIALS AND METHODS
Study population
This study was based on data from the 2010~2012 Korean National Health and Nutrition Examination Survey (KNHANES), a cross-sectional, population-based health examination and survey conducted by the Korea Centers for Disease Control and Prevention with Institutional Review Board approval
7. The survey used multistage, stratified, probability cluster sampling to produce estimated health statistics representative of the noninstitutionalized civilian population of the Republic of Korea. The survey consisted of four components: a health-related interview, a health behavior survey, a health examination, and a nutrition survey. The interview included questions on demographics, socioeconomics, health, and nutrition. The health examination included vital signs, physiological measurements, and basic laboratory tests. We included subjects as AD patients who answered “yes” to the following question: “Have you ever been diagnosed as atopic dermatitis by doctor?.” This study adhered to the tenets of the Declaration of Helsinki for biomedical research involving human subjects. Approval for this study was obtained from the institutional review board/Ethics Committee of Soonchunhyang University Bucheon Hospital (approval number SCHBC 2017-12-014).
Ophthalmic examinations and surveys
Participants underwent ophthalmic interviews, visual acuity measurements, intraocular pressure (IOP) measurements, autorefraction, slit-lamp examination, and fundus photography for their ophthalmic examinations. A past history of ophthalmic surgery and a family history of any ophthalmic disease were obtained from the interviews. The presence of dry eye disease was determined by following question: “Have you ever been diagnosed with dry eye disease by a doctor?” Available responses were “yes” or “no”. If a participant answered “yes,” he or she was classified as having clinical diagnosis of dry eye disease. A slit-lamp examination was performed to detect anterior segment abnormalities, evaluate the status of cataracts, and measure anterior chamber depth using the Van Herick method. The standard Lens Opacities Classification System (LOCS) III was used to categorize the type of cataract. Through a comparison with standard photographs, cataracts were categorized as cortical (LOCS III score ≥2), nuclear (LOCS III score ≥4 for nuclear opalescence or nuclear color), posterior subcapsular (PSC; LOCS III score ≥2), or mixed (more than one type of cataract per eye). The spherical equivalent was calculated as the sphere +1/2 cylinder. The IOP was measured once per eye from right to left with a Goldmann applanation tonometer (BQ-900; Haag-Streit, Bern, Switzerland) by a trained ophthalmologist. A digital non-mydriatic retina camera (TRC-NW6S; Topcon, Tokyo, Japan) and a Nikon D-80 digital camera (Nikon, Tokyo, Japan) were used to obtain digital fundus images under physiological mydriasis. Horizontal and vertical cup-to-disc ratios were measured from the fundus images, and features of diabetic retinopathy or age-related macular degeneration were checked.
A visual field test with frequency double technology was performed on participants with suspected glaucomatous features of the optic disc or an elevated IOP (≥22 mmHg). Suspected glaucomatous features of the optic disc included (1) a horizontal or vertical cup to disc ratio (VCDR) ≥0.5, (2) presence of optic disc hemorrhage, (3) presence of retinal nerve fiber layer defects, or (4) violation of the ISNT rule (i.e., normal eyes had a neuroretinal rim thickness configuration as follows: inferior>superior> nasal>temporal). Open-angle glaucoma was diagnosed using modified International Society of Geographical and Epidemiological Ophthalmology criteria for the Korean population. The specific diagnostic criteria were as follows: category 1 required both (1) a reliable visual field defect consistent with glaucoma (a fixation error and false-positive error ≤1 and the presence of at least two locations of reduced sensitivity) and (2) glaucomatous optic disc (neuroretinal rim loss with a VCDR or horizontal cup-to-disc ratio ≥0.6, presence of optic disc hemorrhage, presence of retinal nerve fiber layer defects, or asymmetry of the VCDR ≥0.2); category 2, if a visual field test was not available or was unreliable (a fixation error or false-positive error ≥2), required a VCDR ≥0.9, asymmetry of the VCDR ≥0.3, or presence of retinal nerve fiber layer defects and violation of the ISNT rule; category 3 required visual acuity ≤3/60 and IOP >21 mmHg. Subjects with a shallow anterior chamber (a peripheral anterior chamber depth less than or equal to one fourth of the central corneal thickness, indicating angle-closure glaucoma) were excluded.
Other covariates
Demographic and socioeconomic variables, including age, sex, urban or rural residence, income, and education level, were obtained from a health interview. Age was classified into 10-year intervals, income was stratified into quartiles, and education was categorized as elementary school or lower, middle school graduate, high school graduate and college graduate or higher. Regarding the region of residence, the 16 districts of the Republic of Korea were divided into two groups: (1) urban regions: Seoul, Gyeonggi, Busan, Daegu, Incheon, Gwangju, Daejeoun, and Ulsan; and (2) rural regions: Gangwon, Chungbuk, Chungnam, Jeonnam, Jeonbuk, Gyeongbuk, Gyeongnam, and Jeju. Occupation was classified into three groups: (1) white collar, comprising managers, professionals, clerical support workers, and service and sales workers; (2) blue collar, including agriculture, forestry, fishery, craft, and related trade workers; plant and machine operators and assemblers; and simple laborers; and (3) no occupation, including unemployed people, retired people, students, and homemakers. Body mass index (BMI) was calculated as follows: weight (kg)/height (m2). Smoking status was categorized as smoker, ex-smoker, or nonsmoker. Drinking habits were classified according to drinking frequency as following: none, ocassional (less than 2 times per week), and frequent (more than 2 times per week).
Systolic and diastolic blood pressure (BP) were measured three times using a standard mercury sphygmomanometer (Baumanometer; WA Baum, Copiague, NY, USA) in a seated position, and the mean values for systolic and diastolic BP were recorded. Subjects were defined as having hypertension if they had a history of taking antihypertensive medications or had a BP ≥140/90 mmHg. Participants who had been diagnosed with diabetes by physicians or had a fasting glucose level ≥126 mg/dl were defined as having diabetes mellitus, and hypercholesterolemia was defined as having a fasting total cholesterol level ≥240 mg/dl or taking oral lipid-lowering agents.
Statistical analysis
Statistical analyses were performed using SAS for Windows, version 9.3 (SAS Institute, Cary, NC, USA). The PROC SURVEY procedure with sample weights was used to account for a complex sampling design and to produce nationally representative prevalence estimates. We presented the demographic characteristics of the participants according to the presence of glaucoma as either means±standard errors (SEs) or proportions and compared them between groups using the Rao–Scott chi-square test. We performed 4-to-1 matching by adjusting age and sex. Multiple logistic regression analyses were conducted to determine the associations between AD and various ocular disorders. An adjusted model was developed that included occupation and region as potential confounders. Odds ratios (ORs) and 95% confidence intervals (CIs) were also calculated. In all analyses, a two-tailed p<0.05 was considered statistically significant.
Go to :

RESULTS
Of 25,534 participants in the 2010~2012 KNHANES, 10,634 were excluded for the following reasons: <19 years of age (n=5,935), missing values for sample weights (n=3,212), and no responders for questions for AD (n=531), dry eye disease (n=712), cataract (n=53), or glaucoma (n=191). Ultimately 14,900 participants were included in the statistical analyses: 14,598 non-AD subjects and 302 subjects with AD.
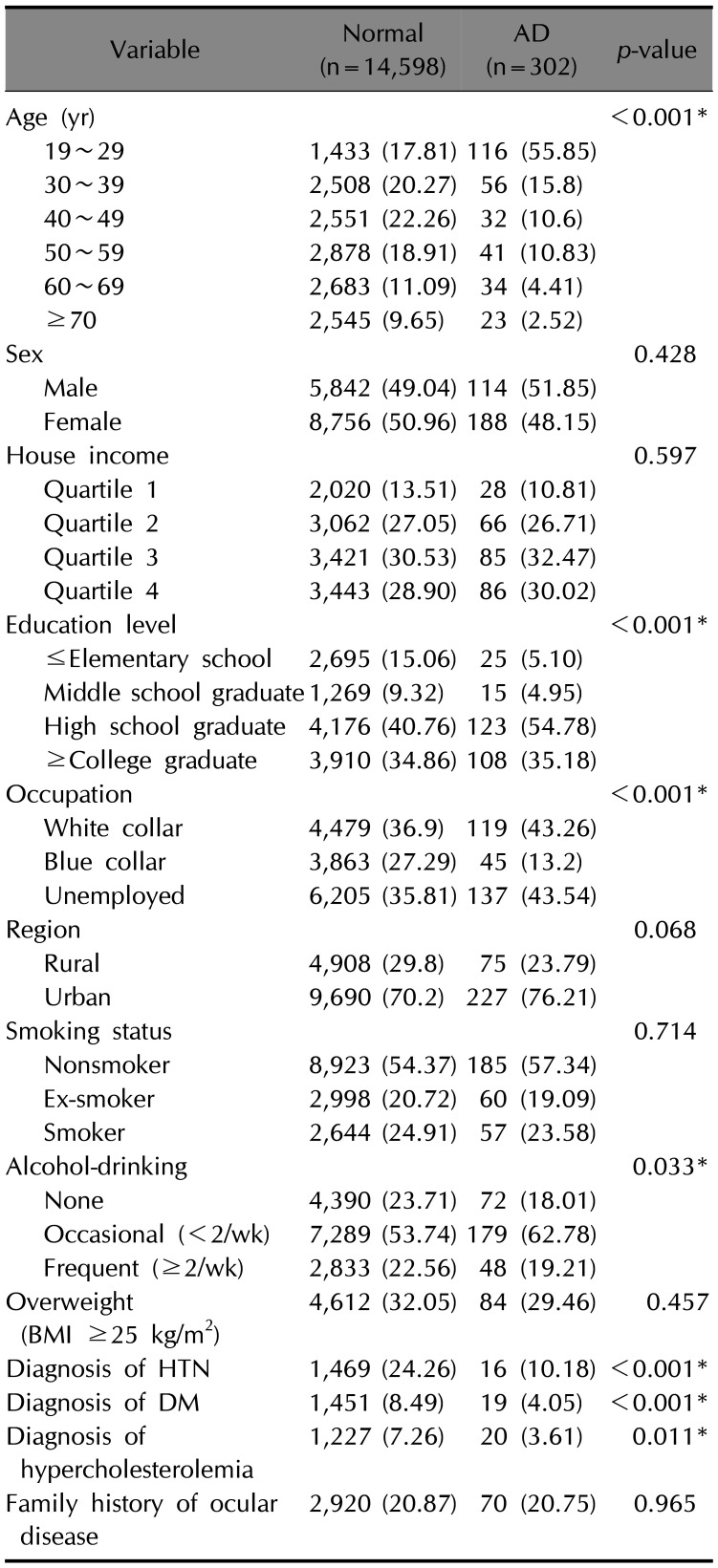
Demographic characteristics of the participants are shown in
Table 1. Participants with AD were significantly younger (
p<0.001); were more highly educated (
p<0.001); were less frequent drinkers (
p=0.033); and had a lower prevalence of hypertension (
p<0.001), diabetes mellitus (
p<0.001), and hypercholesterolemia (
p=0.011) compared to the non-AD group. Significant difference in occupation was observed between non-AD and AD groups (
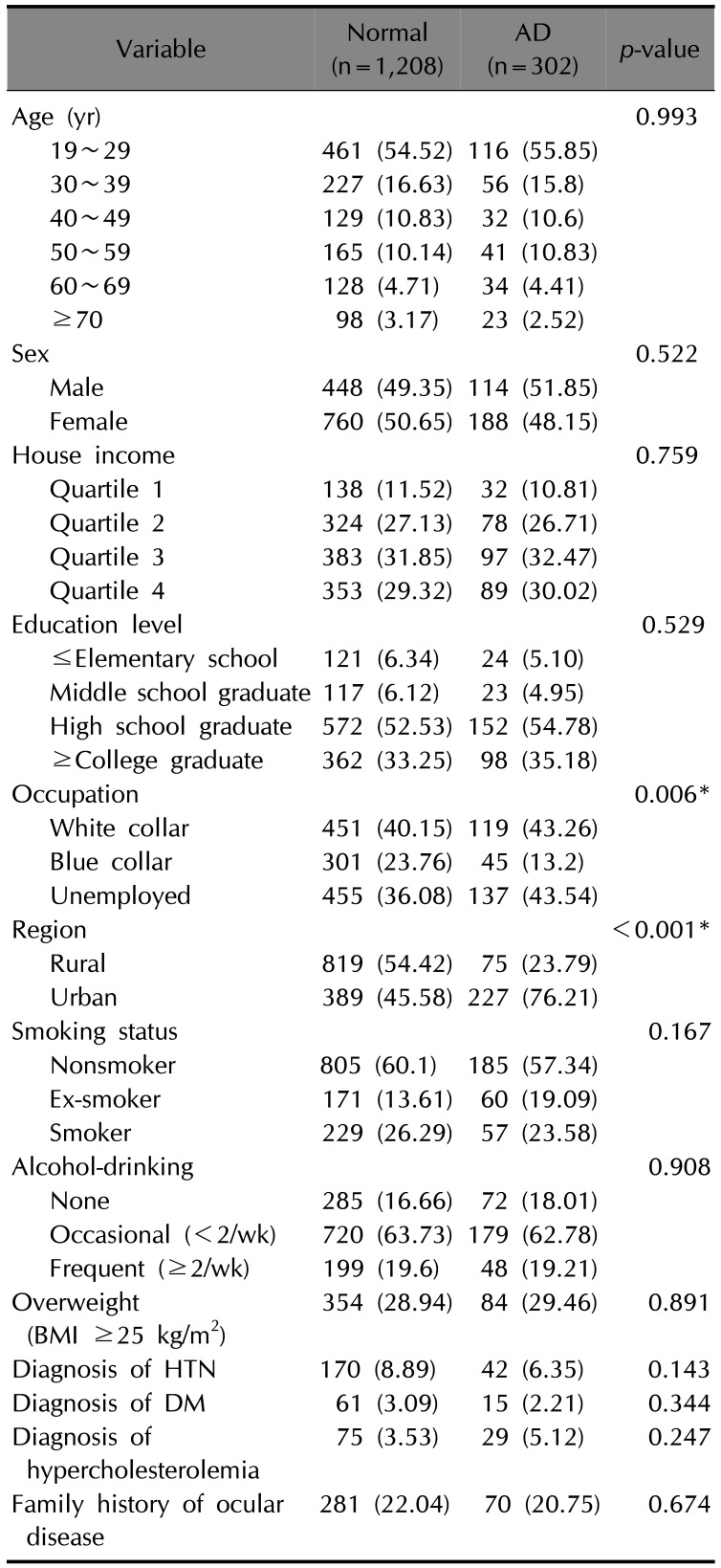
p<0.001). After we performed 4-to-1 matching by adjusting age and sex (
Table 2), the difference in occupation remained significant between non-AD and AD groups (
p=0.006) and AD participants were more likely to live in urban areas (
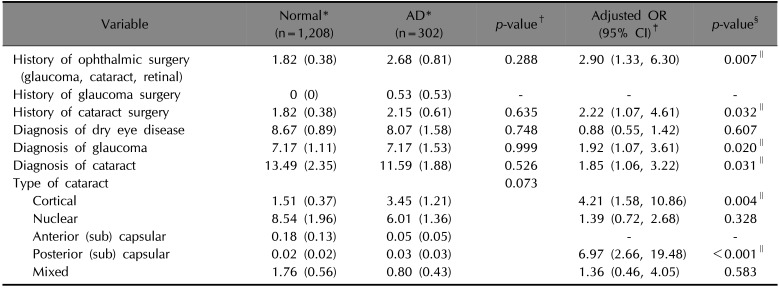
p<0.001). The prevalence and adjusted odd ratios for ocular diseases including dry eye disease, glaucoma, and cataract, and history of ocular surgery in matched participants are shown in
Table 3. There were no significant differences between non-AD and AD groups in the prevalence of ocular disease or history of ophthalmic surgery. Multiple logistic regression analyses were performed to identify associations between AD and ocular disease. After we adjusted for potential confounding factors, a history of ophthalmic surgery (OR, 2.90; 95% CI, 1.33~6.30;
p=0.007), a history of cataract surgery (OR, 2.22; 95% CI, 1.07~4.61;
p=0.032), a diagnosis of glaucoma (OR, 1.92; 95% CI, 1.11~3.32;
p=0.020), and a diagnosis of cataract (OR, 1.85; 95% CI, 1.06~3.22;
p=0.031) were significantly associated with AD. Of the five subtypes of cataracts, the cortical type (OR, 4.21; 95% CI, 1.58~10.86;
p=0.004) and posterior subcapsular type (OR, 6.97; 95% CI, 2.66~19.48;
p<0.001) were significantly associated with AD.
Table 1
Baseline characteristics according to atopic dermatitis in Korean adults
|
Variable |
Normal (n=14,598) |
AD (n=302) |
p-value |
|
Age (yr) |
|
|
<0.001*
|
|
19~29 |
1,433 (17.81) |
116 (55.85) |
|
30~39 |
2,508 (20.27) |
56 (15.8) |
|
40~49 |
2,551 (22.26) |
32 (10.6) |
|
50~59 |
2,878 (18.91) |
41 (10.83) |
|
60~69 |
2,683 (11.09) |
34 (4.41) |
|
≥70 |
2,545 (9.65) |
23 (2.52) |
|
Sex |
|
|
0.428 |
|
Male |
5,842 (49.04) |
114 (51.85) |
|
Female |
8,756 (50.96) |
188 (48.15) |
|
House income |
|
|
0.597 |
|
Quartile 1 |
2,020 (13.51) |
28 (10.81) |
|
Quartile 2 |
3,062 (27.05) |
66 (26.71) |
|
Quartile 3 |
3,421 (30.53) |
85 (32.47) |
|
Quartile 4 |
3,443 (28.90) |
86 (30.02) |
|
Education level |
|
|
<0.001*
|
|
≤Elementary school |
2,695 (15.06) |
25 (5.10) |
|
Middle school graduate |
1,269 (9.32) |
15 (4.95) |
|
High school graduate |
4,176 (40.76) |
123 (54.78) |
|
≥College graduate |
3,910 (34.86) |
108 (35.18) |
|
Occupation |
|
|
<0.001*
|
|
White collar |
4,479 (36.9) |
119 (43.26) |
|
Blue collar |
3,863 (27.29) |
45 (13.2) |
|
Unemployed |
6,205 (35.81) |
137 (43.54) |
|
Region |
|
|
0.068 |
|
Rural |
4,908 (29.8) |
75 (23.79) |
|
Urban |
9,690 (70.2) |
227 (76.21) |
|
Smoking status |
|
|
0.714 |
|
Nonsmoker |
8,923 (54.37) |
185 (57.34) |
|
Ex-smoker |
2,998 (20.72) |
60 (19.09) |
|
Smoker |
2,644 (24.91) |
57 (23.58) |
|
Alcohol-drinking |
|
|
0.033*
|
|
None |
4,390 (23.71) |
72 (18.01) |
|
Occasional (<2/wk) |
7,289 (53.74) |
179 (62.78) |
|
Frequent (≥2/wk) |
2,833 (22.56) |
48 (19.21) |
|
Overweight (BMI ≥25 kg/m2) |
4,612 (32.05) |
84 (29.46) |
0.457 |
|
Diagnosis of HTN |
1,469 (24.26) |
16 (10.18) |
<0.001*
|
|
Diagnosis of DM |
1,451 (8.49) |
19 (4.05) |
<0.001*
|
|
Diagnosis of hypercholesterolemia |
1,227 (7.26) |
20 (3.61) |
0.011*
|
|
Family history of ocular disease |
2,920 (20.87) |
70 (20.75) |
0.965 |

Table 2
Baseline characteristics according to atopic dermatitis in Korean adults after matching for age and sex
|
Variable |
Normal (n=1,208) |
AD (n=302) |
p-value |
|
Age (yr) |
|
|
0.993 |
|
19~29 |
461 (54.52) |
116 (55.85) |
|
30~39 |
227 (16.63) |
56 (15.8) |
|
40~49 |
129 (10.83) |
32 (10.6) |
|
50~59 |
165 (10.14) |
41 (10.83) |
|
60~69 |
128 (4.71) |
34 (4.41) |
|
≥70 |
98 (3.17) |
23 (2.52) |
|
Sex |
|
|
0.522 |
|
Male |
448 (49.35) |
114 (51.85) |
|
Female |
760 (50.65) |
188 (48.15) |
|
House income |
|
|
0.759 |
|
Quartile 1 |
138 (11.52) |
32 (10.81) |
|
Quartile 2 |
324 (27.13) |
78 (26.71) |
|
Quartile 3 |
383 (31.85) |
97 (32.47) |
|
Quartile 4 |
353 (29.32) |
89 (30.02) |
|
Education level |
|
|
0.529 |
|
≤Elementary school |
121 (6.34) |
24 (5.10) |
|
Middle school graduate |
117 (6.12) |
23 (4.95) |
|
High school graduate |
572 (52.53) |
152 (54.78) |
|
≥College graduate |
362 (33.25) |
98 (35.18) |
|
Occupation |
|
|
0.006*
|
|
White collar |
451 (40.15) |
119 (43.26) |
|
Blue collar |
301 (23.76) |
45 (13.2) |
|
Unemployed |
455 (36.08) |
137 (43.54) |
|
Region |
|
|
<0.001*
|
|
Rural |
819 (54.42) |
75 (23.79) |
|
Urban |
389 (45.58) |
227 (76.21) |
|
Smoking status |
|
|
0.167 |
|
Nonsmoker |
805 (60.1) |
185 (57.34) |
|
Ex-smoker |
171 (13.61) |
60 (19.09) |
|
Smoker |
229 (26.29) |
57 (23.58) |
|
Alcohol-drinking |
|
|
0.908 |
|
None |
285 (16.66) |
72 (18.01) |
|
Occasional (<2/wk) |
720 (63.73) |
179 (62.78) |
|
Frequent (≥2/wk) |
199 (19.6) |
48 (19.21) |
|
Overweight (BMI ≥25 kg/m2) |
354 (28.94) |
84 (29.46) |
0.891 |
|
Diagnosis of HTN |
170 (8.89) |
42 (6.35) |
0.143 |
|
Diagnosis of DM |
61 (3.09) |
15 (2.21) |
0.344 |
|
Diagnosis of hypercholesterolemia |
75 (3.53) |
29 (5.12) |
0.247 |
|
Family history of ocular disease |
281 (22.04) |
70 (20.75) |
0.674 |

Table 3
Prevalence and adjusted odds ratios for ocular diseases according to atopic dermatitis in Korean adults after matching for age and sex
|
Variable |
Normal* (n=1,208) |
AD* (n=302) |
p-value†
|
Adjusted OR (95% CI)‡
|
p-value§
|
|
History of ophthalmic surgery (glaucoma, cataract, retinal) |
1.82 (0.38) |
2.68 (0.81) |
0.288 |
2.90 (1.33, 6.30) |
0.007∥
|
|
History of glaucoma surgery |
0 (0) |
0.53 (0.53) |
- |
- |
- |
|
History of cataract surgery |
1.82 (0.38) |
2.15 (0.61) |
0.635 |
2.22 (1.07, 4.61) |
0.032∥
|
|
Diagnosis of dry eye disease |
8.67 (0.89) |
8.07 (1.58) |
0.748 |
0.88 (0.55, 1.42) |
0.607 |
|
Diagnosis of glaucoma |
7.17 (1.11) |
7.17 (1.53) |
0.999 |
1.92 (1.07, 3.61) |
0.020∥
|
|
Diagnosis of cataract |
13.49 (2.35) |
11.59 (1.88) |
0.526 |
1.85 (1.06, 3.22) |
0.031∥
|
|
Type of cataract |
|
|
0.073 |
|
|
|
Cortical |
1.51 (0.37) |
3.45 (1.21) |
4.21 (1.58, 10.86) |
0.004∥
|
|
Nuclear |
8.54 (1.96) |
6.01 (1.36) |
1.39 (0.72, 2.68) |
0.328 |
|
Anterior (sub) capsular |
0.18 (0.13) |
0.05 (0.05) |
- |
- |
|
Posterior (sub) capsular |
0.02 (0.02) |
0.03 (0.03) |
6.97 (2.66, 19.48) |
<0.001∥
|
|
Mixed |
1.76 (0.56) |
0.80 (0.43) |
1.36 (0.46, 4.05) |
0.583 |

Go to :

DISCUSSION
Various ocular diseases are associated with AD, including cataract, retinal detachment, blepharitis, glaucoma, keratoconjunctivitis, and keratoconus
4. This study characterized the possible association between AD and cataract, glaucoma, and dry eye disease in the adult population of the Republic of Korea using data obtained from the KNHANES. The prevalences of dry eye disease, glaucoma, and cataract in adults with AD were estimated to be 8.07%, 7.17%, and 11.59%, respectively in this study. After we adjusted for confounding factors, we found that patients with AD had a higher prevalence of ophthalmic surgery, in particular cataract surgery, compared to the normal control group. Patients with AD had a greater likelihood of having been diagnosed with glaucoma and cataract compared to non-AD subjects. Of the five subtypes of cataracts, cortical and posterior subcapsular cataracts were significantly associated with AD. However, an association between dry eye disease and AD was not found.
Cataract is one of the most well-known ocular complications of AD. Because there are not many well-organized studies, the exact prevalence of cataract in patients with AD is currently unknown, but it varies from 1% to 38%
689. In our study, it was estimated to be 11.59%, which is consistent with previous studies. The pathogenesis of cataract in AD is not definitively known. There may be multiple causes, including direct trauma, inflammatory processes associated with AD, and long-term use of corticosteroids
9101112. Oxidative stress in AD patients has also frequently been suggested as a main contributing factor to developing cataracts
9. A higher level of serum lipid peroxide produced from unsaturated fatty acids and a lower level of superoxide dismutase were observed in AD patients with cataracts compared to AD patients without cataracts
913, irrespective of previous topical corticosteroid treatments
13. Decreased activity by superoxide dismutase, which scavenges reactive oxygen species (ROS) and inhibits the formation of free radicals, has been reported in AD patients with cataracts
913. Elevated serum IgE levels and genetic factors associated with AD may also be associated with the formation of cataracts
14.
Among the many subtypes of cataracts, subcapsular cataracts have typically been reported as being associated with AD. Although the anterior subscapular cataract (ASC) is more specifically related to AD, the PSC is more common in AD patients
31215. In our study, AD was associated with PSCs, consistent with previous studies. However, an association with ASCs could not be determined because the prevalence of ASC was very low. The reason for the low prevalence of both ASC and PSC in this study is probably the large number of patients undergoing early cataract surgery for these two cataract subtypes, because the location of lens opacification is relatively central to the visual axis and thus may impair visual acuity more severely. The significant association between AD and cataract surgery found in this study is consistent with this hypothesis.
The exact pathogenesis of the ASC is still not known. However, the ASC is neither an age-related nor steroid-induced cataract
16. It is probably closely related to AD because AD has been suggested as the most common cause of the ASC
31215. The ASC tends to develop in early childhood
16, and repeated physical trauma due to rubbing and scratching the eyes plays an important role
9. Steroid treatment is the most well-known factor contributing to the formation of the PSC
3691718. This treatment disturbs the normal metabolism of ocular cells, resulting in osmotic failure, thus making lens proteins more vulnerable to oxidative stress
18. Because AD is a chronic inflammatory skin disorder that often requires long-term corticosteroid use, the association between PSC and AD seems reasonable. However, another study reported no differences in the incidence of cataracts between corticosteroid-naive AD patients and AD patients with a history of corticosteroid use, which suggests that the PSC in patients with AD cannot be fully explained by the use of corticosteroids alone
13.
Our study showed that cortical cataracts were also associated with AD. Known risk factors for cortical cataracts include age, female sex, genetic factors, diabetes mellitus, and ultraviolet B light (UVB)
10. In our study, the association between AD and the cortical cataract subtype was present even after we adjusted for age, sex, and diabetes mellitus, which suggests that other factors may have influenced the formation of cortical cataracts. Among these risk factors, the relationship between UVB and cataract has frequently been suggested
101920. Patients with AD may have a greater chance of being exposed to narrowband UVB, because narrowband UVB is widely used in chronic AD treatment. Having unprotected eyes during therapy may increase the risk of UVB exposure among AD patients
9.
The relationship between AD and glaucoma has often been reported since Harris
21 first suggested glaucoma as an adverse effect of glucocorticoid treatment in 1960. Although a recent study from Denmark reported no relationship between glaucoma and AD
4, many studies have reported a positive association between glaucoma and AD, which is consistent with the results of our study. Steroid administration has frequently been suggested as the single most important cause of the development of glaucoma in patients with AD. The elevated IOP induced by the use of corticosteroids may cause optic nerve damage, resulting in glaucoma
322. Recently, an increased inflammatory response in AD was suggested as another possible factor contributing to the development of glaucoma in AD patients. The study reported that inflammatory cytokines such as IL-8 and CCL2 were increased in glaucoma patients with AD
22.
Dry eye disease has been suggested as a related symptom of atopic keratoconjunctivitis (AKC), which is a severe ocular complication in AD patients
523. Abnormal tear functioning, including meibomian gland dysfunction and decreased tear film break-up time, have been suggested as the main pathogenic mechanisms involved in developing irritation and dry eye in patients with AKC
523. Goblet cell loss and conjunctival squamous metaplasia may also contribute to lacrimal film instability in patients with AKC
5. However, whether dry eye syndrome can develop independently from AKC in patients with AD has not been definitively established, and further studies are needed to confirm this relationship. The results of the present study showed no significant association between AD and a diagnosis of dry eye disease by clinicians. One possible reason for this is that the diagnosis of dry eye disease was based on self-report questionnaires; most of the participants with AD may have had a doctor's diagnosis of AKC but not a specific diagnosis of dry eye disease.
The present study has some limitations. First, given the cross-sectional nature of the study, causal relationships between AD and the ocular diseases cannot be definitively established. Second, because the KNHANES is based on self-reported data, there was the potential for incorrect classifications by the participants. Third, although we included several potential confounders, unidentified factors may have contributed to these associations. Fourth, the route of administration, dose, frequency, and duration of corticosteroid treatments are important factors in the pathogenesis of cataract and glaucoma in AD patients
242526, but these data were not provided by KNHANES. Nonetheless, a strength of the present study is that it used data from a nationwide survey that included a large population, which minimizes selection bias. In addition, because few population-based studies have investigated possible associations between ocular disease and AD worldwide, our study may help to confirm and expand previous findings of associations between cataract and glaucoma in patients with AD.
In summary, this is the first study to comprehensively investigate the associations between AD and selected ocular disorders in the Republic of Korea. The results showed that cataract and glaucoma were significantly associated with AD in adult patients. Furthermore, there was a significantly higher prevalence of ophthalmic surgery among AD patients than non-AD patients, which implies that ocular diseases occurring in patients with AD require more frequent surgical interventions. Dermatologists should therefore be aware of ocular manifestations in patients with AD and should recommend regular screening of these patients for the early detection of ocular disorders.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download