Abstract
Background
Osteoporosis is a geriatric disease with diminished bone density. The increase in the number of patients and medical expenses due to a global aging society are recognized as problems. Bone loss is the most common symptom of bone disease, not only osteoporosis but Paget's disease, rheumatoid arthritis, multiple myeloma, and other diseases. The main cause of this symptoms is excessive increase in the number and activity of osteoclasts. Osteoclasts are multinucleated giant cells that can resorb bone. They are differentiated and activation from monocytes/macrophages in the presence of macrophage colony-stimulating factor and receptor activator of nuclear factor-κB ligand (RANKL).
Methods
The effect of extract of Flavoparmelia sp. (EFV), a genus of lichenized fungi within the Parmeliaceae, on the differentiation of bone marrow-derived macrophages (BMMs) into osteoclasts was examined by phenotype assay and the cell cytotoxicity was evaluated by cell counting kit-8. The osteoclast differentiation-related genes and proteins were investigated by real-time polymerase chain reaction and immunoblotting. The functional activity of osteoclast in response to EFV treatment was evaluated by an Osteo Assay plate.
Results
In this study, we found that EFV, a genus of lichenized fungi within the Parmeliaceae, inhibited osteoclast formation. And we investigated its inhibitory mechanism. EFV reduced RANKL-mediated osteoclast formation and activation by inhibiting expression of nuclear factor of activated T cells 1, a key factor of osteoclastogenesis.
Bones are an organ that changes over time during the process of production, growth, and absorption over a lifetime. Approximately 10% of an adult bone is replaced with new bone each year.[1] The bone density is highest in people in their 20s to 30s and decreases gradually after that. In women, the bone mineral density decreases rapidly during the first 5 years of menopause.[2] Excessive bone loss is a major cause of osteoporosis, which is becoming a public health problem because of the increased frequency of osteoporotic fractures in elderly people.[3] Osteoporosis is an age-related disorder that is characterized by bone loss and deterioration of the bone structure, particularly affecting postmenopausal women.[4]
Bone homeostasis/remodeling is important for maintaining the bone mass and quality. This process requires a balanced action of bone-resorbing osteoclasts and bone-forming osteoblasts.[5] Typical bone diseases, such as osteoporosis, Paget's disease, and rheumatoid arthritis, have the symptoms of decreased bone mass because of the increased number and activity of osteoclasts.[6] Osteoclasts are large multinucleated cells (MNCs) that remove the old/weakened bones by acid decalcification and proteolytic degradation.[7] They are differentiated from the monocyte-macrophage of a hematopoietic lineage. Osteoclast formation is controlled by 2 cytokines, macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL), which are secreted by osteoblasts/activated T cells.[8] M-CSF acts on osteoclast precursor cells to induce signaling related to cell survival and promotes the expression of RANKL receptor, RANK.[9] RANKL is also known as the tumor necrosis factor-related activation-induced cytokine or osteoclast-differentiation factor. Binding of RANKL and RANK ultimately leads to the formation of osteoclasts by increasing the expression of nuclear factor of activated T cells 1 (NFATc1), a key transcription factor for osteoclastogenesis.[10]
Lichen is a symbiotic relationship between fungi (mycobiont) and photosynthetic organisms (photobionts) that use each other's metabolites to generate a range of secondary metabolites.[1112] Their metabolites have been discovered over the past decade and are being assessed to find new bioactive compounds.[1314] Recently, various species of lichen were collected and bioactive extracts were obtained. In addition, the extracts of Flavoparmelia sp. (EFV) inhibited osteoclast formation. This study investigated the mechanisms by which EFV inhibits osteoclast formation.
Flavoparmelia sp. was collected from Peru in May of 2017 during a field trip of San Tuario, Muruhuay, Acobamba, Junin organized by Prof. Rebeca Magdalena Pavlich Herrera at Peruvian University Cayetano Heredia, Peru. The field trip was conducted in the frame of an internal joint program between Korea and Peru, supported by Korea National Research Foundation. The field studies did not involve endangered or protected species. Duplicates were deposited at the Korean Lichen and Allied Bioresource Center in the Korean Lichen Research Institute, Sunchon National University (SNCU), Korea. The air-dried Flavoparmelia sp. (10 g) were extracted twice with 2 L methanol at room temperature for 48 hr using sonication. The extract was filtered then concentrated under vacuum at 40℃ using a rotary evaporator. The extract was subjected to high performance liquid chromatography (HPLC) analyses (LC-20A; Shimadzu, Kyoto, Japan) on a YMC-Pack™ ODS-A (150×3.9 mm I.D.; YMC, Kyoto, Japan) reverse-phase column containing fully end-capped C18 material (particle size, 5 µm; pore size, 12 nm). Elution was performed at a flow rate of 1 mL/min under the following conditions before subsequent injection: column temperature, 40℃; and solvent system, methanol: water:phosphoric acid (80:20:1, v/v/v). The analyses were monitored using a photodiode array detector (SPD-M20A; Shimadzu) over the range, 190 to 800 nm, throughout the HPLC run. The observed peaks were scanned between 190 and 400 nm.
This study was conducted in strict accordance with the recommendations contained in the Standard Protocol for Animal Study of SCNU. The protocol was approved by the SCNU Institutional Animal Care and Use Committee (IACUC) with Permit No. SCNU IACUC 2016-06. All efforts were made to minimize suffering.
All cells were cultured in a 5% CO2 at 37℃. The culture medium was replaced with fresh medium every 3 days. Bone marrow cells (BMCs) were isolated from the femurs and tibias of 5-week-old male ICR mice (n=2; RaonBio Inc., Yongin, Korea). The BMCs were incubated with 10 ng/mL M-CSF (PeproTech, Rocky Hill, NJ, USA) for 16 hr in α-minimum essential medium (MEM; Thermo Fisher Scientific Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific Inc.) and 100 U/mL penicillin/streptomycin (10% α-MEM) on a 10 cm culture dish. The non-adherent cells were cultured with 30 ng/mL M-CSF in 10% α-MEM on a 10 cm Petri dish. After 3 days, the adhered cells were harvested and used as bone marrow-derived macrophages (BMMs). The BMMs were cultured with 10 ng/mL RANKL (R&D Systems, Minneapolis, MN, USA) and 30 ng/mL M-CSF in 10% α-MEM for 4 days in the presence of the vehicle (0.1% dimethyl sulfoxide [DMSO]) or EFV.
The adherent cells were fixed with 10% formaldehyde for 5 min, permeabilized with 0.1% Triton X-100 for 10 min, and incubated with a tartrate-resistant acid phosphatase (TRAP)-staining solution (Sigma-Aldrich, St. Louis, MO, USA) at room temperature for 10 min. The TRAP-positive cells stained red and stained cells with 3 or more nuclei were counted as mature osteoclasts.
BMMs were cultured with 30 ng/mL M-CSF in 10% α-MEM in the presence of the vehicle (0.1% DMSO) or EFV. After 3 days, the cell viability was assessed using a cell counting kit-8 (CCK-8; Dojindo Molecular Technologies, Kumamoto, Japan) according to the manufacturer's protocols.
Real-time PCR was performed, as described elsewhere.[15] BMMs were cultured with 10 ng/mL RANKL and 30 ng/mL M-CSF in 10% α-MEM for the indicated days in the presence of vehicle (0.1% DMSO) or EFV. The primer sets for real-time PCR were designed (Table 1) using the online primer3 program.[16] The total RNA was obtained using the TRIzol reagent (Thermo Fisher Scientific Inc.) according to the manufacturer's protocol. First-strand cDNA was modified using a moloney murine leukemia virus cDNA Synthesis kit (Enzynomics, Daejeon, Korea) according to the manufacturer's instructions. Quantitative PCR (qPCR) was performed using the TOPreal qPCR 2×PreMIX (Bio-Rad, Hercules, CA, USA) in a Real-Time PCR Detection System (Bio-Rad). The relative levels of the tested genes were normalized to the level of glyceraldehyde-3-phosphate dehydrogenase and the data were analyzed using the 2−ΔΔCT method.[17]
Western blotting was performed, as described previously.[18] BMMs were incubated in the same manner as real-time PCR assays. The cells were washed with phosphate-buffered saline and lysed in a lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 5 mM ethylenediaminetetraacetic acid, 1% Triton X-100, 1 mM sodium fluoride, 1 mM sodium vanadate, and 1% deoxycholate) supplemented with 1 mM phenylmethylsulfonyl fluoride (Bio Basic Inc., Amherst, NY, USA). The lysates were centrifuged at 20,000×g for 13 min at 4℃ and the supernatant containing the proteins was collected. The proteins were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Millipore Corporation, Billerica, MA, USA). The membranes were blocked with 5% skim milk for 1 hr at room temperature and incubated overnight at 4℃ with the primary antibody. They were then incubated with the secondary antibody conjugated to horseradish peroxidase for 2 hr at room temperature. The membranes were developed with CLAROTM Mucho (Bio-D, Gwangmyeong, Korea) using a LAS-4000 luminescent image analyzer (Fuji Photo Film Co. Ltd., Tokyo, Japan).
A bone pit formation assay was performed, as described elsewhere.[19] BMMs were seeded on an Osteo Assay plate (24 well plate; Corning, Tewksbury, MA, USA) at a density of 3×105 cells/well and cultured with 10 ng/mL RANKL and 30 ng/mL M-CSF in the presence of vehicle (0.1% DMSO) or EFV. After 4 days, the cells were removed completely with 5% sodium hypochlorite for 5 min, and the pit area was then observed by optical microscopy (magnification, ×50; Leica Microsystems, Wetzlar, Germany) and measured by ImageJ software (National Institutes of Health, Bethesda, MD, USA).
HPLC analysis of the methanol extract of Flavoparmelia sp. showed that galbinic acid was the main component of the extract (Fig. 1). The retention time (Rt=2.242) and the maximum absorption wavelengths (λmax) of the UV-spectra (the insert in Fig. 1) of the peak confirmed that the compound was well matched with galbinic acid.
The potential role of EFV in osteoclastogenesis was evaluated by examining the effects of EFV on the ability of RANKL to differentiate BMMs. BMMs were cultured with 0.1% DMSO (vehicle) or EFV (0, 1, 3, and 10 µg/mL) for 4 days in the presence of 10 ng/mL RANKL and 30 ng/mL M-CSF. Red stained TRAP-positive cells were induced by RANKL, but EFV reduced this induction (Fig. 2A). Moreover, EFV inhibited the number of TRAP-positive MNCs (nuclei ≥ 3) in a dose-dependent manner and the formation of MNCs was inhibited almost completely at 10 µg/mL (Fig. 2B).
To determine if the anti-osteoclastogenesis efficacy of EFV is due to cytotoxicity, cell viability analysis was performed with a CCK-8 in BMMs. The BMMs were incubated with 0.1% DMSO (vehicle) or EFV (0, 1, 3, and 10 µg/mL) for 3 days in the presence of 30 ng/mL M-CSF. EFV had no cytotoxic effects on the BMMs at the indicated concentration in this study (Fig. 2C).
The effects of EFV on gene expression of NFATc1, a major transcriptional factor of osteoclast differentiation, were examined. Real-time PCR showed that RANKL gradually increased the mRNA level of NFATc1, but EFV decreased the transcriptional level of NFATc1 significantly (Fig. 3A). Moreover, EFV also reduced the transcriptional levels of TRAP, dendritic cell-specific transmembrane protein (DC-STAMP), and cathepsin K, the osteoclast differentiation marker genes regulated by NFATc1 (Fig. 3B–D).
In the previous experiment, EFV reduced the mRNA expression of NFATc1. Hence, this study examined whether EFV affected the protein level of NFATc1 by western blotting. The protein level of NFATc1 was increased significantly by RANKL but was reduced dramatically by EFV (Fig. 4). This suggests that EFV inhibits the translational expression of NFATc1 and suppresses osteoclast formation.
Experiments were conducted to determine if the osteoclast formation inhibited by EFV also affects bone resorption. The osteoclasts formed wide pit areas on the bone slice, but EFV reduced the pit areas markedly (Fig. 5).
Lichens are a complex organism, in which algae or cyanobacteria (photobionts) live among the filaments of various fungi (mycobiont).[11] In addition, a lichen thallus often contains diverse assemblages of microfungi and microorganisms.[20] The combination of these various organisms allows the lichen to generate and use various secondary metabolites,[12] such as alkaloids,[21] peptides,[22] and terpenes.[2324] Their metabolites have been studied to discover new bioactive compounds [2526] and their biological activity is also being revealed.[272829]
Osteoporosis is a representative geriatric disease that is becoming a major concern because of the aging population. The disease results in an increased risk of bone fractures by lowering the strength of the bone because the number and activity of osteoclasts are increased due to a range of causes.[30] In addition, investigations have shown that the number of fracture patients due to osteoporosis and the cost of treatment are increasing.[313233] Therefore, it is necessary to study various means of preventing and treating bone diseases, such as osteoporosis. Osteoclasts are MNCs arising from hematopoietic stem cells/macrophage lineage. Their differentiation and function is controlled by M-CSF and RANKL, which are produced in mature osteoblasts and stromal cells.[734] M-CSF is involved in the growth, survival, proliferation, and differentiation of both hematopoietic and non-hematopoietic cells.[3536] In particular, it promotes the expression of the RANKL receptor, RANK, in osteoclastic progenitor cells.[37] RANKL is the most important cytokine during the osteoclast differentiation process.[1037] The RANKL-RANK signaling pathway activates NFATc1. As a result, the osteoclast precursor is differentiated into TRAP-positive MNCs (mature osteoclast).
In vitro screening experiments with the extract of various lichens extracts on RANKL-mediated osteoclast differentiation were performed prior to this study. The results showed that EFV inhibited the osteoclast differentiation. Subsequently, this study examined the inhibitive mechanism of EFV against osteoclast formation. First, EFV was treated at concentrations of 0, 1, 3, and 10 µg/mL during osteoclast differentiation to select the optimal concentration of EFV in this study. EFV significantly reduced osteoclast formation at concentrations ≥3 µg/mL and did not exhibit cytotoxicity even at concentrations of 10 µg/mL. The cells were treated with EFV at a concentration of 10 µg/mL, which showed optimal efficacy in the experiment, and then examined by real-time PCR and western blotting to identify the anti-osteoclastogenic mechanism.
NFATc1 is a master transcription factor on osteoclastogenesis and is activated by the RANKL signaling pathway. In this study, EFV inhibited RANKL-mediated mRNA and the protein expression level of NFATc1. In addition, EFV reduced the levels of TRAP, DC-STAMP, and cathepsin K expression, which are osteoclast formation and activation-related molecules, by decreasing NFATc1.[3738394041] And we confirmed that RANKL-induced bone resorption was decreased by EFV in vitro. This means that EFV inhibits and reduces the formation and activity of osteoclasts. Overall, these results suggest that EFV had a substance that affected osteoclast differentiation and function, and this substance reduced the expression of NFATc1, a key molecule during osteoclastogenesis.
Figures and Tables
 | Fig. 1High performance liquid chromatography (HPLC) profiling of methanol extract from Flavoparmelia sp. methanol extract of Flavoparmelia sp. was analyzed by HPLC with YMC-Pack ODS-A reversed-phase C18 column. The ultraviolet–visible-spectra of the maximum absorption wavelengths (λmax) of galbinic acid are present in the insert. By comparing both the retention time and λmax with the standard, the main compound of the extract was identified to be galbinic acid. The molecular structure of galbinic acid is also presented in the inset. |
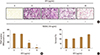 | Fig. 2The extracts of Flavoparmelia sp. (EFV) inhibited osteoclast differentiation. (A) Bone marrow-derived macrophages (BMMs) were cultured with 10 ng/mL receptor activator of nuclear factor-κB ligand (RANKL) and 30 ng/mL macrophage colony-stimulating factor (M-CSF) for 4 days in the presence of the vehicle (0.1% dimethyl sulfoxide [DMSO]) or the indicated concentrations of EFV. The cells were fixed in 3.7% formalin, permeabilized with 0.1% Triton X-100, and stained with tartrate-resistant acid phosphatase (TRAP) solution. (B) TRAP-positive multinucleated cells (3 or more nuclei) were counted as osteoclasts. (C) BMMs were cultured with 30 ng/mL M-CSF for 3 days in the presence of the vehicle (0.1% DMSO) or the indicated concentrations of EFV. The effects of EFV on the BMMs viability were assessed using a cell counting kit-8. *P<0.001 (n=3). |
 | Fig. 3Effects of the extracts of Flavoparmelia sp. (EFV) on receptor activator of nuclear factor-κB ligand (RANKL)-mediated mRNA expressions of nuclear factor of activated T cells 1 (NFATc1). The bone marrow-derived macrophages were treated with the vehicle (0.1% DW) or EFV (10 µg/mL) and macrophage colony-stimulating factor (30 ng/mL) for 1 hr and then RANKL (10 ng/mL) were treated at the indicated times. The total RNA was then isolated using the TRIzol reagent, and the mRNA expression levels were evaluated by performing real-time polymerase chain reaction. (A) NFATc1, (B) dendritic cell-specific transmembrane protein (DC-STAMP), (C) tartrate-resistant acid phosphatase (TRAP), and (D) cathepsin K. Glyceraldehyde-3-phosphate dehydrogenase was used as the internal control. *P<0.05. **P<0.01. ***P<0.001. |
 | Fig. 4The extracts of Flavoparmelia sp. (EFV) abolishes receptor activator of nuclear factor-κB ligand (RANKL)-mediated protein expression of nuclear factor of activated T cells 1 (NFATc1). Bone marrow-derived macrophages were pretreated with the vehicle (0.1% dimethyl sulfoxide) or EFV (10 µg/mL) and macrophage colony-stimulating factor (30 ng/mL) for 1 hr prior to RANKL (10 ng/mL) stimulation for the indicated times. The cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and Western blotting was performed with anti-NFATc1, and anti-actin antibodies as indicated. |
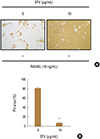 | Fig. 5The extracts of Flavoparmelia sp. (EFV) inhibited bone resorption by receptor activator of nuclear factor-κB ligand (RANKL)-induced osteoclasts. (A) Bone marrow-derived macrophages were plated on an Osteo Assay Plate and treated with 30 ng/mL macrophage colony-stimulating factor and 10 ng/mL RANKL in the presence of 10 µg/mL of EFV. After 4 days of culture, the cells attached to the Osteo Assay plate were removed and photographed under an optical microscope. (B) Pit areas were quantified using the ImageJ program. *P<0.001 (n=3). |
Acknowledgments
This research was supported by the R&D Program for Forest Science Technology (2017024A00-1919-BA01) provided by Korea Forest Service (Korea Forestry Promotion Institute), Sunchon National University Research (2018-0190), and the National Research Foundation of Korea (NRF-2016K1A3A1A12953760).
References
1. Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000; 21:115–137.

2. Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008; 93:861–868.

3. Kanis JA, Johnell O, Oden A, et al. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001; 12:989–995.

4. Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014; 142:155–170.

5. Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res. 2005; 20:177–184.

7. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003; 423:337–342.

9. Kansara M, Teng MW, Smyth MJ, et al. Translational biology of osteosarcoma. Nat Rev Cancer. 2014; 14:722–735.

10. Takayanagi H, Kim S, Koga T, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002; 3:889–901.

11. Spribille T, Tuovinen V, Resl P, et al. Basidiomycete yeasts in the cortex of ascomycete macrolichens. Science. 2016; 353:488–492.

12. Kellogg JJ, Raja HA. Endolichenic fungi: a new source of rich bioactive secondary metabolites on the horizon. Phytochem Rev. 2017; 16:271–293.

13. Chen GD, Chen Y, Gao H, et al. Xanthoquinodins from the endolichenic fungal strain Chaetomium elatum. J Nat Prod. 2013; 76:702–709.

14. Li XB, Zhou YH, Zhu RX, et al. Identification and biological evaluation of secondary metabolites from the endolichenic fungus Aspergillus versicolor. Chem Biodivers. 2015; 12:575–592.

15. Kim KJ, Yeon JT, Choi SW, et al. Decursin inhibits osteoclastogenesis by downregulating NFATc1 and blocking fusion of pre-osteoclasts. Bone. 2015; 81:208–216.

16. Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000; 132:365–386.

17. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408.

18. Kim H, Kim KJ, Yeon JT, et al. Placotylene A, an inhibitor of the receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation, from a Korean sponge Placospongia sp. Mar Drugs. 2014; 12:2054–2065.

19. Kim KJ, Lee Y, Hwang HG, et al. Betulin suppresses osteoclast formation via down-regulating NFATc1. J Clin Med. 2018; 7:154.

20. Girlanda M, Isocrono D, Bianco C, et al. Two foliose lichens as microfungal ecological niches. Mycologia. 1997; 89:531–536.

21. Li XB, Li L, Zhu RX, et al. Tetramic acids and pyridone alkaloids from the endolichenic fungus tolypocladium cylindrosporum. J Nat Prod. 2015; 78:2155–2160.

22. Wu W, Dai H, Bao L, et al. Isolation and structural elucidation of proline-containing cyclopentapeptides from an endolichenic Xylaria sp. J Nat Prod. 2011; 74:1303–1308.

23. Wijeratne EM, Bashyal BP, Liu MX, et al. Geopyxins A-E, ent-kaurane diterpenoids from endolichenic fungal strains Geopyxis aff. majalis and Geopyxis sp. AZ0066: structure-activity relationships of geopyxins and their analogues. J Nat Prod. 2012; 75:361–369.

24. Wu YH, Chen GD, Wang CX, et al. Pericoterpenoid A, a new bioactive cadinane-type sesquiterpene from Periconia sp. J Asian Nat Prod Res. 2015; 17:671–675.

25. Frisvad JC, Andersen B, Thrane U. The use of secondary metabolite profiling in chemotaxonomy of filamentous fungi. Mycol Res. 2008; 112:231–240.

26. Parrot D, Jan S, Baert N, et al. Comparative metabolite profiling and chemical study of Ramalina siliquosa complex using LC-ESI-MS/MS approach. Phytochemistry. 2013; 89:114–124.

27. Kim GS, Ko W, Kim JW, et al. Bioactive α-pyrone derivatives from the endolichenic fungus dothideomycetes sp. EL003334. J Nat Prod. 2018; 81:1084–1088.

28. Kim JW, Ko W, Kim E, et al. Anti-inflammatory phomalichenones from an endolichenic fungus Phoma sp. J Antibiot (Tokyo). 2018; 71:753–756.

29. Kim KJ, Jeong MH, Lee Y, et al. Effect of usnic acid on osteoclastogenic activity. J Clin Med. 2018; 7:345.

30. Golob AL, Laya MB. Osteoporosis: screening, prevention, and management. Med Clin North Am. 2015; 99:587–606.
31. Blume SW, Curtis JR. Medical costs of osteoporosis in the elderly Medicare population. Osteoporos Int. 2011; 22:1835–1844.

32. Svedbom A, Hernlund E, Ivergård M, et al. Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos. 2013; 8:137.

33. Wade SW, Strader C, Fitzpatrick LA, et al. Estimating prevalence of osteoporosis: examples from industrialized countries. Arch Osteoporos. 2014; 9:182.

34. Yasuda H, Shima N, Nakagawa N, et al. A novel molecular mechanism modulating osteoclast differentiation and function. Bone. 1999; 25:109–113.

36. Tanaka S, Takahashi N, Udagawa N, et al. Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J Clin Invest. 1993; 91:257–263.

37. Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007; 7:292–304.

38. Minkin C. Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int. 1982; 34:285–290.

39. Kim B, Kim HH, Lee ZH. α-Tocopheryl succinate inhibits osteolytic bone metastasis of breast cancer by suppressing migration of cancer cells and receptor activator of nuclear factor-κB ligand expression of osteoblasts. J Bone Metab. 2018; 25:23–33.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download