Abstract
Purpose
Assessing asthma control is important for proper management, and various indices for objective assessment of asthma control, such as fractional exhaled nitric oxide (FeNO) and asthma control test (ACT) score have been proposed. Recently, bronchodilator response (BDR) was reported as a marker of poor asthma control in adults. This study aimed to assess the usefulness of BDR as a biomarker for childhood asthma.
Methods
A total of 305 children diagnosed with asthma were included. Spirometry with bronchodilator test was done at the time of diagnosis and about 14 months after asthma treatment. All children were evaluated by childhood asthma control test (c-ACT) and FeNO after asthma treatment. The patients were divided into 2 groups according to BDR results: the positive and negative BDR groups. Various biomarkers for asthma control, such as c-ACT, FeNO and changes of forced expiratory volume in 1 second (FEV1), were compared between the 2 groups.
Results
Of the 305 patients, 143 (46.9%) were positive and 162 (53.1%) were negative for BDR. The BDR-positive group showed lower FEV1. In the BDR positive group, FEV1 was significantly increased after asthma treatment, especially in children with airflow limitation which was defined as below 80% of FEV1 or atopy. In atopic children, BDR showed a significant negative correlation with c-ACT and a positive correlation with FeNO.
Go to : 
References
1. Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995; 8:483–91.

2. Hong SJ, Lee MS, Sohn MH, Shim JY, Han YS, Park KS, et al. Self-report-ed prevalence and risk factors of asthma among Korean adolescents: 5-year follow-up study, 1995–2000. Clin Exp Allergy. 2004; 34:1556–62.

3. Sol IS, Kim YH, Kim SY, Choi SH, Kim JD, Kim BO, et al. Prescription patterns and burden of pediatric asthma in Korea. Allergy Asthma Immunol Res. 2019; 11:280–90.

4. Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007; 119:817–25.

5. Juniper EF, Gruffydd-Jones K, Ward S, Svensson K. Asthma Control Questionnaire in children: validation, measurement properties, interpretation. Eur Respir J. 2010; 36:1410–6.

6. Chipps B, Zeiger RS, Murphy K, Mellon M, Schatz M, Kosinski M, et al. Longitudinal validation of the Test for Respiratory and Asthma Control in Kids in pediatric practices. Pediatrics. 2011; 127:e737–47.

7. Lu M, Wu B, Che D, Qiao R, Gu H. FeNO and asthma treatment in children: a systematic review and metaanalysis. Medicine (Baltimore). 2015; 94:e347.
8. Petsky HL, Kew KM, Chang AB. Exhaled nitric oxide levels to guide treatment for children with asthma. Cochrane Database Syst Rev. 2016; 11:CD011439.

9. American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 171:912–30.
10. Melosini L, Dente FL, Bacci E, Bartoli ML, Cianchetti S, Costa F, et al. Asthma control test (ACT): comparison with clinical, functional, and biological markers of asthma control. J Asthma. 2012; 49:317–23.

11. Heffler E, Crimi C, Campisi R, Sichili S, Nicolosi G, Porto M, et al. Bronchodilator response as a marker of poor asthma control. Respir Med. 2016; 112:45–50.

12. Galant SP, Morphew T, Newcomb RL, Hioe K, Guijon O, Liao O. The relationship of the bronchodilator response phenotype to poor asthma control in children with normal spirometry. J Pediatr. 2011; 158:953–9.e1.

13. Kim YH, Kim KW, Baek J, Park HB, Kim H, Song KJ, et al. Usefulness of impulse oscillometry and fractional exhaled nitric oxide in children with Eosinophilic bronchitis. Pediatr Pulmonol. 2013; 48:221–8.

14. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–38.
15. Ferrer Galván M, Javier Alvarez Gutiérrez F, Romero Falcón A, Romero Romero B, Sáez A, Medina Gallardo JF. Is the bronchodilator test an useful tool to measure asthma control? Respir Med. 2017; 126:26–31.

16. The Global Initiative for Asthma. 2018 Update GINA report, global strategy for asthma management and prevention [Internet]. The Global Initiative for Asthma; [cited 2019 Mar 14]. Available from:. https://ginasth-ma.org/wp-content/uploads/2018/04/wms-GINA-2018-report-V1.3-002.pdf.
17. Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006; 117:549–56.

18. Ulrik CS, Frederiksen J. Mortality and markers of risk of asthma death among 1,075 outpatients with asthma. Chest. 1995; 108:10–5.

19. Pongracic JA, Krouse RZ, Babineau DC, Zoratti EM, Cohen RT, Wood RA, et al. Distinguishing characteristics of difficult-to-control asthma in inner-city children and adolescents. J Allergy Clin Immunol. 2016; 138:1030–41.

20. Kim YH, Sol IS, Yoon SH, Kim MJ, Kim KW, Sohn MH, et al. Association of extended nitric oxide parameters with bronchial hyperresponsiveness and bronchodilator response in children with asthma. J Breath Res. 2017; 11:046003.

21. Parker AL. Airway reactivity is a determinant of bronchodilator responsiveness after methacholine-induced bronchoconstriction. J Asthma. 2004; 41:671–7.

22. Goleva E, Hauk PJ, Boguniewicz J, Martin RJ, Leung DY. Airway remodeling and lack of bronchodilator response in steroid-resistant asthma. J Allergy Clin Immunol. 2007; 120:1065–72.

23. Tantisira KG, Fuhlbrigge AL, Tonascia J, Van Natta M, Zeiger RS, Strunk RC, et al. Bronchodilation and bronchoconstriction: predictors of future lung function in childhood asthma. J Allergy Clin Immunol. 2006; 117:1264–71.

24. Bauer S, Park HN, Seo HS, Kim JE, Song DJ, Park SH, et al. Assessment of bronchodilator responsiveness following methacholine-induced bronchoconstriction in children with asthma. Allergy Asthma Immunol Res. 2011; 3:245–50.

25. Mogensen I, Alving K, Dahlen SE, James A, Forsberg B, Ono J, et al. Fixed airflow obstruction relates to eosinophil activation in asthmatics. Clin Exp Allergy. 2019; 49:155–62.

26. Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009; 180:59–99.
Go to : 
 | Fig. 1.Comparison of results of pulmonary function test at asthma diagnosis and after asthma treatment. According to absence or presence of bronchodilator response (BDR) (A), more or less than 80% of FEV1, among presence of BDR (B), absence or presence of airway hyperresponsiveness, among presence of BDR and less than 80% of FEV1 (C), absence or presence of atopy, among presence of BDR and less than 80% of FEV1 (D). FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; PEF, peak expiratory flow; FEF25%–75%, forced expiratory flow from 25% to 75% of vital capacity; Pre BD, prebronchodilator; Post BD, postbronchodilator. * P<0.001, † P<0.05. |
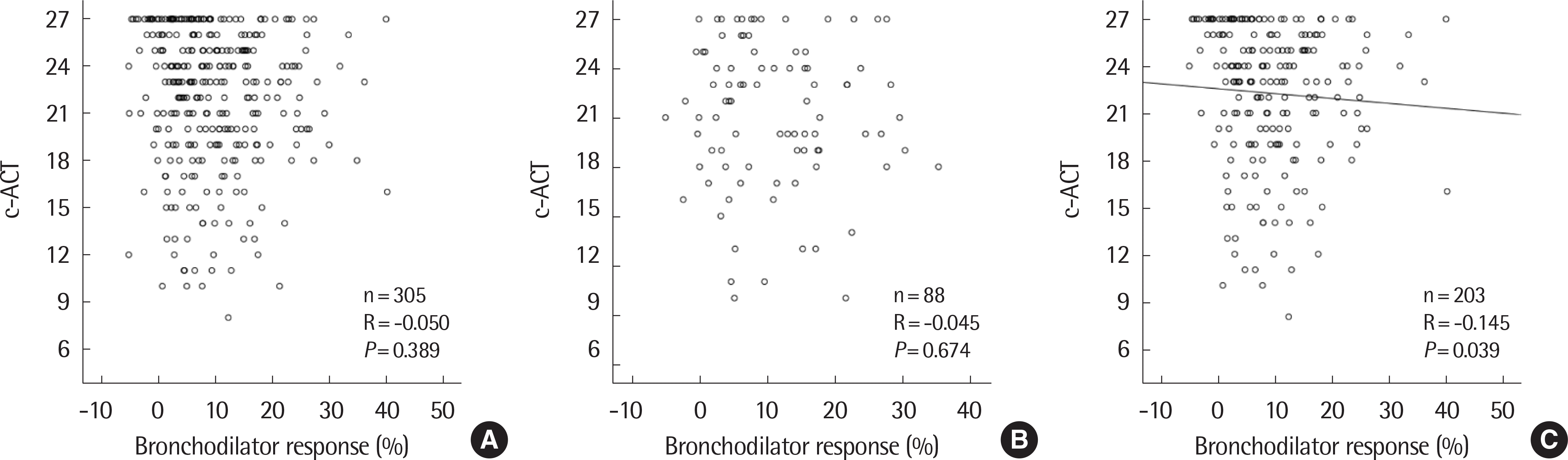 | Fig. 2.Correlation between bronchodilator response and childhood asthma control test (c-ACT) using scatter plot in total (A), nonatopic (B), and atopic children (C). |
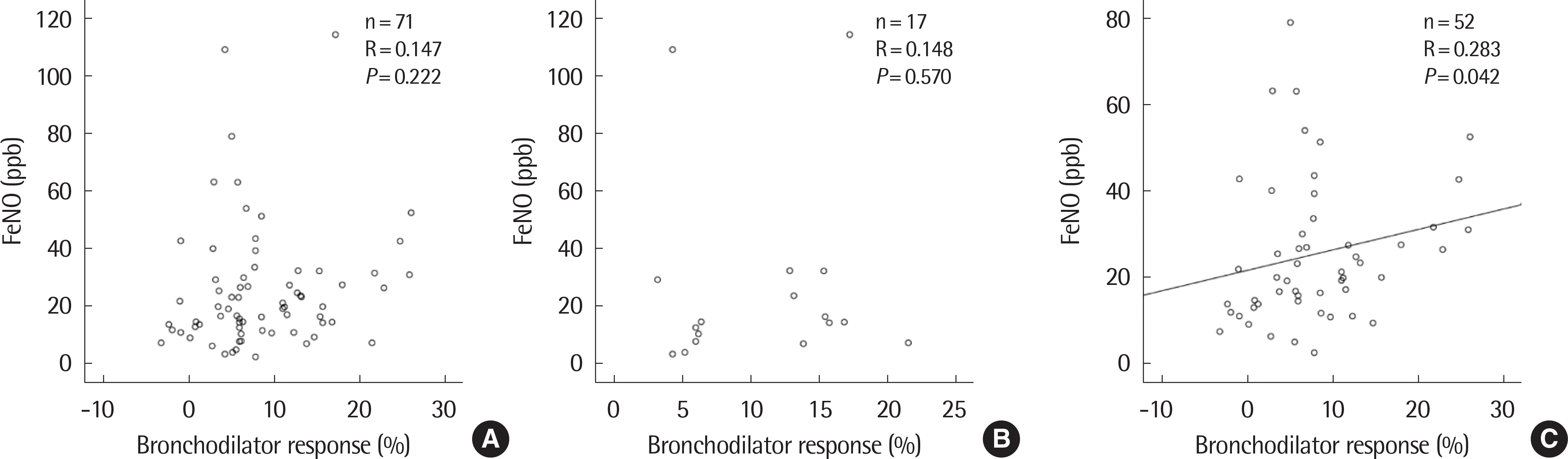 | Fig. 3.Correlation between bronchodilator response and fractional exhaled nitric oxide (FeNO) using scatter plot in total (A), nonatopic (B), and atopic children (C). |
Table 1.
Subject characteristics according to bronchodilator response in baseline pulmonary function test
Values are presented as number (%) or mean±standard deviation.
BDR, bronchodilator response; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; PEF, peak expiratory flow; FEV1/FVC, forced expiratory volume in 1 second/Forced vital capacity; FEF25%–75%, forced expiratory flow from 25% to 75% of vital capacity.
Table 2.
Association of c-ACT and atopy with changes in bronchodilator response after asthma management




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download