Abstract
Purpose
Multiple virus infections may affect clinical severity. We investigated the effect of coinfection of respiratory syncytial virus (RSV) and influenza virus with other respiratory viruses on clinical severity.
Methods
Data from 634 samples of a single tertiary hospital between September 2014 and April 2015 were analyzed for clinical characteristics (fever duration and O2 need, steroid use, and ICU care) between single infection and coinfection of RSV (n=290) and influenza virus (n=74) with 16 common respiratory viruses from hospitalized children.
Results
The RSV coinfection group (n=109) (3.1±2.7 days) showed significantly longer fever duration than the RSV single infection group (n=181) (2.6±2.6 days) (P=0.04), while there was no difference in O2 need, steroid use or ICU care in the 2 groups. The influenza coinfection group (n=38) showed significantly higher O2 need than the influenza single infection group (n=36) (21.1% vs. 5.6%, P=0.05), while there was no difference in fever duration between the 2 groups.
Go to : 
References
1. Henrickson KJ. Lower respiratory viral infections in immunocompetent children. Adv Pediatr Infect Dis. 1994; 9:59–96.
2. Kuypers J, Wright N, Ferrenberg J, Huang ML, Cent A, Corey L, et al. Comparison of realtime PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006; 44:2382–8.

3. Kuiken T, Fouchier R, Rimmelzwaan G, Osterhaus A. Emerging viral infections in a rapidly changing world. Curr Opin Biotechnol. 2003; 14:641–6.

4. Choi EH, Lee HJ, Kim SJ, Eun BW, Kim NH, Lee JA, et al. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000–2005. Clin Infect Dis. 2006; 43:585–92.

5. Harada Y, Kinoshita F, Yoshida LM, Minh le N, Suzuki M, Morimoto K, et al. Does respiratory virus coinfection increases the clinical severity of acute respiratory infection among children infected with respiratory syncytial virus? Pediatr Infect Dis J. 2013; 32:441–5.

6. Martin ET, Kuypers J, Wald A, Englund JA. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respir Viruses. 2012; 6:71–7.

7. Rho EJ, Jin YM, Chung EH, Rheem I, Kim JK. The prevalence of respiratory viral infection in exacerbation of asthma in hospitalized children. Korean J Asthma Allergy Clin Immunol. 2007; 27:241–7.
8. Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med. 2008; 121:258–64.

9. Aberle JH, Aberle SW, Pracher E, Hutter HP, Kundi M, Popow-Kraupp T. Single versus dual respiratory virus infections in hospitalized infants: impact on clinical course of disease and interferon-gamma response. Pediatr Infect Dis J. 2005; 24:605–10.
10. Nagayama Y, Sakurai N, Yamamoto K, Honda A, Makuta M, Suzuki R. Isolation of Mycoplasma pneumoniae from children with lower-respiratory-tract infections. J Infect Dis. 1988; 157:911–7.

11. Denny FW, Clyde WA Jr. Acute lower respiratory tract infections in nonhospitalized children. J Pediatr. 1986; 108(5 Pt 1):635–46.

12. Matsumoto K. Clinical manifestation and prognosis of respiratory syncytial virus infection in infants. Arerugi. 1992; 41:1679–86.
13. Ahn KM, Chung SH, Chung EH, Koh YJ, Nam SY, Kim JH, et al. Clinical characteristics of acute viral lower respiratory tract infections in hospitalized children in Seoul, 1996–1998. J Korean Med Sci. 1999; 14:405–11.

14. Machablishvili A, Tsereteli D, Zakhashvili K, Karseladze I, Imnadze P. Clinical and epidemiological characterization of influenza A/H1N1PDM and B among hospitalized children, Georgia, season 2010–2011. Georgian Med News. 2017; 265:71–8.
15. Pinky L, Dobrovolny HM. Coinfections of the respiratory tract: viral competition for resources. PLoS One. 2016; 11:e0155589.

16. Gonzalez AJ, Ijezie EC, Balemba OB, Miura TA. Attenuation of influenza A virus disease severity by viral coinfection in a mouse model. J Virol. 2018; 92(23):pii:. e00881–18.

17. Crowe JE Jr. Respiratory syncytial virus. Kliegman RM, Stanton BF, St. Geme JW III, Schor NF, Behrman RE, editors. Nelson textbook of pediatrics. 20th ed.Philadelphia (PA): Elsevier Saunders;2016. p. 1606–9.

18. Esper FP, Spahlinger T, Zhou L. Rate and influence of respiratory virus coinfection on pandemic (H1N1) influenza disease. J Infect. 2011; 63:260–6.

19. Skjerven HO, Megremis S, Papadopoulos NG, Mowinckel P, Carlsen KH, Lødrup Carlsen KC, et al. Virus type and genomic load in acute bronchiolitis: severity and treatment response with inhaled adrenaline. J Infect Dis. 2016; 213:915–21.

Go to : 
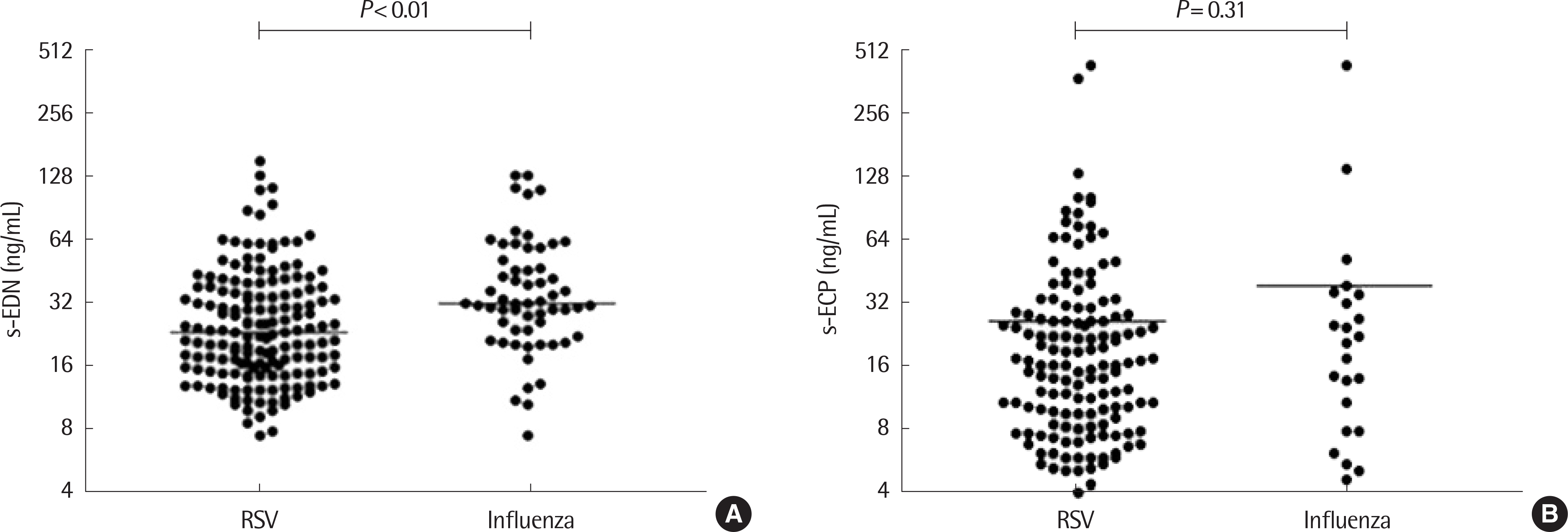 | Fig. 1.Comparison of serum eosinophil-derived neurotoxin (s-EDN) (A) and serum eosinophil cationic protein (s-ECP) (B) between respiratory syncytial virus (RSV) and Influenza groups. |
Table 1.
Baseline characteristics of study subjects
Table 2.
Duration of fever and respiratory difficulties in single infection and coinfection groups
Table 3.
Duration of fever and respiratory difficulties according to level of viral load




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download