Abstract
Purpose
To describe cases of exposed hydroxyapatite (HA) implants wrapped with the synthetic dura substitute Neuro-Patch treated via simple Neuro-Patch removal.
Methods
The medical records of seven patients who experienced exposure of their HA implant were reviewed. All patients had been enucleated and implanted with HA wrapped with Neuro-Patch. For treatment, Neuro-Patch was removed to the greatest extent possible. After applying local anesthesia with lidocaine, blunt dissection was performed to separate the conjunctiva and Neuro-Patch via the site of exposure. Pressure was applied to the remaining Neuro-Patch with forceps and removed with scissors.
Results
Neuro-Patch was visible at the area of exposure in all patients. No surgery beyond initial Neuro-Patch removal was necessary in six of the seven patients. In five cases, the exposed area began to heal rapidly after Neuro-patch removal without primary closure of the defect. In one case, the Neuro-Patch material and all necrotic tissue was removed aggressively due to inflammation around the orbital implant. Lastly, an infection was noted in one case, prompting complete removal of the Neuro-Patch–wrapped HA implant.
Hydroxyapatite (HA) is a porous non-toxic calcium-phosphate salt complex with high biocompatibility that is both strong and lightweight. Following the introduction of HA by Perry in 1985 [12], it has become widely used following enucleation or evisceration because of its excellent cosmetic and functional properties [12]. Indeed, the porous structure of HA allows for fibrovascular tissue ingrowth, which reduces migration and extrusion. However, one of the disadvantages of HA is that the extraocular muscles may become directly attached to the surface. Due to the rough surface properties of HA, these attachments may in turn lead to HA exposure [3].
Various wrapping materials have been studied as a way to allow extraocular muscles to attach to HA implants in order to prevent complications such as exposure. Examples of wrapping materials include banked sclera, autogenous fascia lata, cadaver dura mater, and synthetic materials [456]. These materials act as a barrier that protect the anterior part of the orbital soft tissues against the rough surface of HA. Banked sclera and cadaver dura mater carry the risk of disease transmission if donor tissues are not properly screened and processed, including human immunodeficiency virus, Creutzfeldt-Jakob disease, and hepatitis [78]. On the other hand, autogenous fascia lata has no associated risk of infectious disease transmission, but functional and cosmetic problems can occur at the donor site.
The synthetic dura substitute Neuro-Patch (Aesculap AG, Tuttlingen, Germany) is a non-absorbable, microporous, non-woven material consisting of cleaned aliphatic polyester urethane. Neuro-Patch is commonly used in neurosurgery for cases involving dural defects or cerebrospinal f luid decompression. Neuro-Patch is also used as a wrapping material for orbital implants because it is relatively inexpensive, easy to obtain, and allows for fibrovascular tissue ingrowth [9]. In the present study, we describe seven cases of exposure of HA implants wrapped with Neuro-Patch that were successfully treated by simple Neuro-Patch removal or total implant removal. In cases involving simple Neuro-Patch removal, exposed HA spontaneously healed without the need for primary closure of the conjunctiva.
This retrospective case review-based study was approved by the institutional review board of Severance Hospital, Yonsei University College of Medicine, Seoul, Korea (4-2018-0696). This study conformed to the Declaration of Helsinki and written informed consent was obtained. The medical records of patients who visited our clinic for HA exposure between February 2016 and January 2018 were reviewed. We identified all patients who had been enucleated, given an HA implant wrapped with Neuro-Patch, and presented to our outpatient clinic with an exposed implant.
Neuro-Patch was initially removed to the greatest extent possible through the exposed area. A lidocaine epinephrine solution (Xylocaine 2%, AstraZeneca, London, United Kingdom) was injected at the conjunctiva and deep tissues around the exposed area. After blunt dissection to separate the conjunctiva and Neuro-Patch via the site of exposure, Neuro-Patch was pulled with forceps and removed with scissors. If the implant was severely infected, the implant was removed together with Neuro-Patch. Ofloxacin ointment was applied at the site of exposure, which was then patched. Patients were prohibited from wearing ocular prostheses, and instead used a plastic conformer for 1 to 2 months until the exposed implant was covered with a conjunctival layer. If the exposure was located at the medial or lateral canthal aspect of the implant, the corner of the conformer was shaved by an ocularist using a hand drill to achieve a round shape.
Seven patients with exposure of an implant that had been previously wrapped with Neuro-Patch were retrospectively collated, and the data derived from these patients are presented in Table 1. The age of patients ranged from 11 to 80 years, two were male, and five were female. Four patients had undergone enucleation surgery due to choroidal melanoma, two experienced eye trauma prior to enucleation, and one patient had undergone secondary HA implantation due to anophthalmos. The duration of the interval between orbital implantation and exposure ranged from 4 to 15 years.
In all patients, Neuro-Patch was visible at the site of exposure. In six of the seven patients (cases 1 to 6) no additional surgery was necessary other than Neuro-Patch removal. In cases 1 to 5, Neuro-Patch was putatively removed via the exposed area and the exposed HA began to heal rapidly without any primary closure of the defect. Conjunctival epithelium began to grow and migrate over the exposed HA as early as 1 week after surgery, and the exposure had disappeared completely by the last follow-up (Fig. 1A–1E).
In case 4, although the overlying conjunctival tissues were relatively thinner than surrounding tissues, full growth of the fibrovascular tissue over the area of HA exposure was evident (Fig. 1D). The patient in case 6 visited the clinic complaining of copious discharge from the socket, orbital pain, and recurrent incidence of granulation tissues (Fig. 1F). The Neuro-Patch was removed—at first partially—via the exposed area at an outpatient clinic in the same manner as in cases 1 to 5; however, there was incomplete resolution of symptoms. Subsequent computed tomography revealed a severe inf lammatory reaction around the entire HA implant (Fig. 2A), although bacterial cultures were negative. Under general anesthesia, all of the Neuro-Patch as well as necrotic tissues around the orbital implant were aggressively removed, resulting in complete resolution of exposure and inflammation (Fig. 2B). Removal of the implant was not deemed necessary at any point throughout the follow-up period.
The Neuro-Patch material was not strongly attached to the HA implant surface in cases 1 to 6, and instead was easily dethatched. Symptoms of discharge and foreign body sensation rapidly disappeared after the procedure. The follow-up periods in cases 1 to 6 ranged from 16 to 29 months, with a median of 22 months. As of the last follow-up, there had been no re-exposure of the implant in cases 1 to 6.
In case 7, an infection of the HA by pseudomonas was identified on bacterial culture (Fig. 3A–3C). A combined procedure was subsequently performed in which the Neuro-Patch-wrapped HA implant was completely removed followed by dermal fat grafting under general anesthesia. Postoperatively, none of the previously reported symptoms including pain, discharge, and granulation tissue occurred during the follow-up period.
HA is widely used as an orbital implant following enucleation, usually along with a wrapping material. Human-derived wrapping materials such as banked sclera and cadaver dura mater have associated risks of transmission of infection from donor tissue [1011]. Thus, several studies have been performed to identify an ideal wrapping material to replace human-derived material for HA implants. Such materials must be biocompatible, inexpensive, sterile, resistant to infection, and easy to produce. Examples of synthetic materials that have been investigated include polyglycolic acid mesh and polyglactin mesh [121314].
Neuro-Patch is one of the synthetic wrapping materials originally manufactured as a dura substitute [15]. According to its product description, the microporous structure of Neuro-Patch allows for immediate inward migration of fibroblasts and anchoring of the graft in tissue by collagen without an accompanying inflammatory reaction. However, there have been several documented neurosurgery cases where associated complications such as necrosis, tissue infection, and inflammation occurred after predominant use of Neuro-Patch [1617].
Heimann et al. [18] observed marked inflammatory reactions involving infiltration by giant foreign body cells, macrophages, plasma cells, and neutrophilic/eosinophilic granulocytes in the micropores of Neuro-Patch inserted in the orbit, but only a very small number of fibroblasts. They also noted weak and thin adhesions between wrapping material and orbital connective tissue. In these cases, it may be that the Neuro-Patch blocked passage of fibroblasts into HA, resulting in reduced vascularization of the HA micropores and adhesion to both Tenon's capsule and conjunctiva. Similarly, we noted that the Neuro-Patch was not closely adhered to the HA implant in our patients, but rather was almost entirely separated from the HA surface. In all cases, the Neuro-Patch wrapping material had blocked microvasculature and fibrous tissue growth into the HA implant.
Exposure of an HA implant is a dangerous complication, and various methods have been applied to solve the problem. Dermis fat grafting or oral mucosa grafting with HA drilling are the current standard treatments for HA exposure [19]. However, in the cases described in this study, complete healing of the area of exposure was achieved after simple Neuro-Patch removal. Specifically, we found that Neuro-Patch was easily detached and removed via the exposed area, and within a few days of partial removal of Neuro-Patch fragments without any primary closure, natural healing of the dehiscence was achieved. In most of the small sized exposure cases, surgery was performed in the outpatient clinic rather than the operating room, and no additional procedures or surgery were required.
Based on our findings, we recommend early intervention by way of simple removal of Neuro-Patch as soon as a small exposure is discovered. This simple approach is cost effective and may facilitate rapid recovery from symptoms. However, in cases involving severe inflammation, aggressive removal of Neuro-Patch is required. In the case in our study where this was required, removal of Neuro-Patch without implant removal was sufficient.
In conclusion, we retrospectively collated and reviewed several cases of HA exposure associated with Neuro-Patch wrapping. Surprisingly, we found that the Neuro-Patch wrapping had blocked fibrovascular tissue growth into HA implants, rather than protecting HA surfaces from anterior soft tissues. Our results suggest that this wrapping material may also hinder implant vascularization. Small areas of exposure of Neuro-Patch were easily managed by simple removal of Neuro-Patch in an outpatient clinical setting, which was both time and cost-effective. Primary closure was not necessary for small areas of exposure. Thus, most cases of HA exposure in a wrapped implant can be successfully treated by simple removal if detected and managed early. However, removal should be considered if the implant is severely infected.
Figures and Tables
 | Fig. 1Anophthalmic sockets in cases 1 to 6. (A) Case 1, (B) case 2, (C) case 3, (D) case 4, (E) case 5, and (F) case 6. The upper images in panels show areas of exposed Neuro-Patch of various sizes and lower images show well healed areas of former exposure after Neuro-Patch removal. The formerly exposed areas remained well healed at the last follow-up examination in all six cases. Written informed consent from the patient was obtained. |
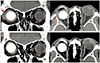 | Fig. 2Case 6. (A) Preoperative computed tomography showing inflammation around the orbital implant (arrow). (B) Postoperative computed tomography showing remarkable improvement of the inflammation around the orbital implant. Written informed consent from the patient was obtained. |
References
3. Jordan DR, Klapper SR. Wrapping hydroxyapatite implants. Ophthalmic Surg Lasers. 1999; 30:403–407.
4. Lee V, Subak-Sharpe I, Hungerford JL, et al. Exposure of primary orbital implants in postenucleation retinoblastoma patients. Ophthalmology. 2000; 107:940–945.
5. Shields CL, Shields JA, De Potter P. Hydroxyapatite orbital implant after enucleation. Experience with initial 100 consecutive cases. Arch Ophthalmol. 1992; 110:333–338.

6. Shields JA, Shields CL, De Potter P. Enucleation technique for children with retinoblastoma. J Pediatr Ophthalmol Strabismus. 1992; 29:213–215.

7. Yamada S, Aiba T, Endo Y, et al. Creutzfeldt-Jakob disease transmitted by a cadaveric dura mater graft. Neurosurgery. 1994; 34:740–743.

8. Mehta JS, Franks WA. The sclera, the prion, and the ophthalmologist. Br J Ophthalmol. 2002; 86:587–592.

9. Raul JS, Godard J, Arbez-Gindre F, Czorny A. Use of polyester urethane (Neuro-Patch) as a dural substitute. Prospective study of 70 cases. Neurochirurgie. 2003; 49(2-3 Pt 1):83–89.
10. Seiff SR, Chang JS Jr, Hurt MH, Khayam-Bashi H. Polymerase chain reaction identification of human immunodeficiency virus-1 in preserved human sclera. Am J Ophthalmol. 1994; 118:528–530.

11. Simonds RJ, Holmberg SD, Hurwitz RL, et al. Transmission of human immunodeficiency virus type 1 from a seronegative organ and tissue donor. N Engl J Med. 1992; 326:726–732.

12. Jordan DR, Ells A, Brownstein S, et al. Vicryl-mesh wrap for the implantation of hydroxyapatite orbital implants: an animal model. Can J Ophthalmol. 1995; 30:241–246.
13. Jordan DR, Allen LH, Ells A, et al. The use of Vicryl mesh (polyglactin 910) for implantation of hydroxyapatite orbital implants. Ophthalmic Plast Reconstr Surg. 1995; 11:95–99.

14. Oestreicher JH, Liu E, Berkowitz M. Complications of hydroxyapatite orbital implants. A review of 100 consecutive cases and a comparison of Dexon mesh (polyglycolic acid) with scleral wrapping. Ophthalmology. 1997; 104:324–329.
15. Gudmundsson G, Sogaard I. Complications to the use of vicryl-collagen dural substitute. Acta Neurochir (Wien). 1995; 132:145–147.

16. Malliti M, Page P, Gury C, et al. Comparison of deep wound infection rates using a synthetic dural substitute (neuro-patch) or pericranium graft for dural closure: a clinical review of 1 year. Neurosurgery. 2004; 54:599–603.

17. El Majdoub F, Lohr M, Maarouf M, et al. Transmigration of fibrino-purulent inflammation and malignant cells into an artificial dura substitute (Neuro-Patch): report of two cases. Acta Neurochir (Wien). 2009; 151:833–835.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download