This article has been
cited by other articles in ScienceCentral.
Abstract
Purpose
To investigate the clinical features and surgical outcomes of encapsulated bleb excision with collagen matrix implantation performed in patients with failed Ahmed glaucoma valve (AGV) implantation.
Methods
Eighteen eyes of 18 patients underwent encapsulated bleb excision and collagen matrix implantation. Patients were divided into two groups by reference to intraocular pressure (IOP) after preoperative ocular massage: group 1, patients who exhibited substantial IOP reductions; and group 2, patients who did not show substantial changes in IOP. Needling was conducted in group 2. The clinical features of the two groups were compared, including IOP changes after ocular massage and needling, AGV status, and surgical outcomes 6 months after surgery.
Results
The mean preoperative IOP among the 18 patients was 30.6 ± 5.7 mmHg. After ocular massage, the IOPs decreased by 22 and 26 mmHg in the two patients in group 1 and the 16 patients in group 2 showed a mean IOP reduction of 1.6 ± 2.2 mmHg (p = 0.013). IOPs decreased after needling in group 2 (range, 6 to 30 mmHg; p < 0.001). Fibrovascular tissue ingrowth into the AGV was observed in the two patients in group 1 and the same ingrowth was observed in 10 of the 16 patients in group 2. Six months after surgery the mean IOP among the 18 patients decreased significantly (19.1 ± 3.2 mmHg, p < 0.001). There was no significant difference in the mean postoperative IOP at 6 months between group 1 (14.0 ± 2.8 mmHg) and group 2 (19.8 ± 2.6 mmHg, p = 0.052).
Conclusions
Encapsulated bleb excision with collagen matrix implantation resulted in a significant IOP-lowering effect 6 months after surgery. Fibrovascular ingrowth into the AGV was common but did not seem to be a major cause of AGV implantation failure.
Go to :

Keywords: Bleb excision, Collagen matrix, Encapsulated bleb, Glaucoma, Intraocular pressure
Glaucoma, the most common form of chronically progressive optic neuropathy, is a principal cause of complete blindness [
1]. The first choice for treating glaucoma is medication. However, surgical treatment should be considered if the side effects of medication are too severe or if intraocular pressure (IOP) is not controllable with medication. Trabeculectomy and glaucoma drainage device implantation are conventional surgical options for glaucoma. Traditionally, glaucoma drainage device implantation has been considered when a previous trabeculectomy was unsuccessful, if there was widespread conjunctival scarring due to previous surgery, or if the success rate of trabeculectomy was expected to decrease due to refractory glaucoma, such as uveitis-related glaucoma or neovascular glaucoma [
23456]. However, recent studies have shown that the success rate of glaucoma drainage device implantation is comparable to trabeculectomy, and that the incidence of complications related to surgery is lower for implantation of a glaucoma drainage device [
7]. The Tube Versus Trabeculectomy Study [
8] reported 5-year postoperative outcomes of 107 patients treated with Baerveldt glaucoma implants (group 1) and 105 patients treated with trabeculectomy (group 2). The mean IOP and the numbers of IOP-lowering eye drops used were the same between the two groups. The failure rates of surgery were 29.8% (group 1) and 46.9% (group 2) (
p = 0.002). Glaucoma drainage devices are more commonly implanted because the indications for this treatment are expanding [
9101112].
Glaucoma drainage devices allow outflow of aqueous humor from a tube inserted into the anterior chamber to the subtenon space around the ocular equatorial area. The body connected to the rear outlet of the tube prevents closure of the tube outlet by surrounding tissue and forms a large retention area beneath the bleb, which is helpful in long-term IOP control. The reported surgical success rate after implantation of glaucoma drainage devices is 68% to 87% at 12 to 24 months after surgery, decreasing by approximately 10% every year [
4131415]. If the IOP increases again after surgery, medication, ocular massage, or needling with or without subconjunctival injection of an antimetabolic agent can be performed [
16]. If the IOP is not controlled using these treatments, additional secondary glaucoma drainage device implantation, cyclophotocoagulation, or encapsulated bleb revision may be performed [
231117]. There have been some reports regarding treatment of patients after implantation failure of a glaucoma drainage device, but details on methodology and surgical results of encapsulated bleb revision are limited [
1718].
This study evaluated the surgical effects of encapsulated bleb excision and collagen matrix implantation in glaucoma patients whose IOP was not controlled by conservative treatment after failure of an implanted Ahmed glaucoma valve (AGV; New World Medical, Rancho Cucamonga, CA, USA), and to determine whether there is a relationship between preoperative clinical features and status of the AGV during surgery.
Materials and Methods
This retrospective study was approved by the institutional review board of Chungnam National University Hospital (CNUH 2018-07-018) which waived the requirement for informed consent from participants. It was conducted in accordance with all relevant requirements of the Declaration of Helsinki. Patients with glaucoma who underwent encapsulated bleb excision and collagen matrix implantation for failed AGV implantation were consecutively enrolled in our glaucoma clinic from August 11, 2014 to January 31, 2018.
Failed AGV implantation was defined as an uncontrolled IOP >20 mmHg after the maximum tolerable medical therapy, and in patients expecting and/or experiencing progression of glaucomatous optic neuropathy. The previous AGV implantation was performed by one of several glaucoma surgeons, including surgeons at other hospitals. All AGV implantations were in the superotemporal quadrant. All excisions of encapsulated blebs and collagen matrix implantations were performed by a single glaucoma surgeon. During the study period, 18 eyes of 18 patients were included. For patients who were scheduled to undergo surgery, the IOP was measured using a Goldmann applanation tonometer at the last outpatient treatment (1 day to 2 weeks before surgery), and ocular massage was performed. With eyes closed, gentle digital pressure was applied to the globe for 10 seconds, avoiding the superotemporal quadrant to minimize the risk that the tube would contact the cornea. Immediately after ocular massage, the IOP was measured once more. For patients without a considerable drop in IOP after ocular massage, needling was performed under slit lamp microscopy. After instillation of proparacaine 0.5% (w/v) and povidone iodine 5% (w/v) eyedrops, patients were instructed to look down in the direction of the inferonasal quadrant. A bent 30-G needle with the bevel in the up position was attached to a 1-mL syringe and the subconjunctival space was entered adjacent to the bleb. Once inside, the needle was advanced to penetrate the bleb wall. Aqueous humor outflow immediately expanded the subconjunctival space and we then measured the IOP. Ofloxacin 0.3% (w/v) eyedrops were prescribed prior to surgery.
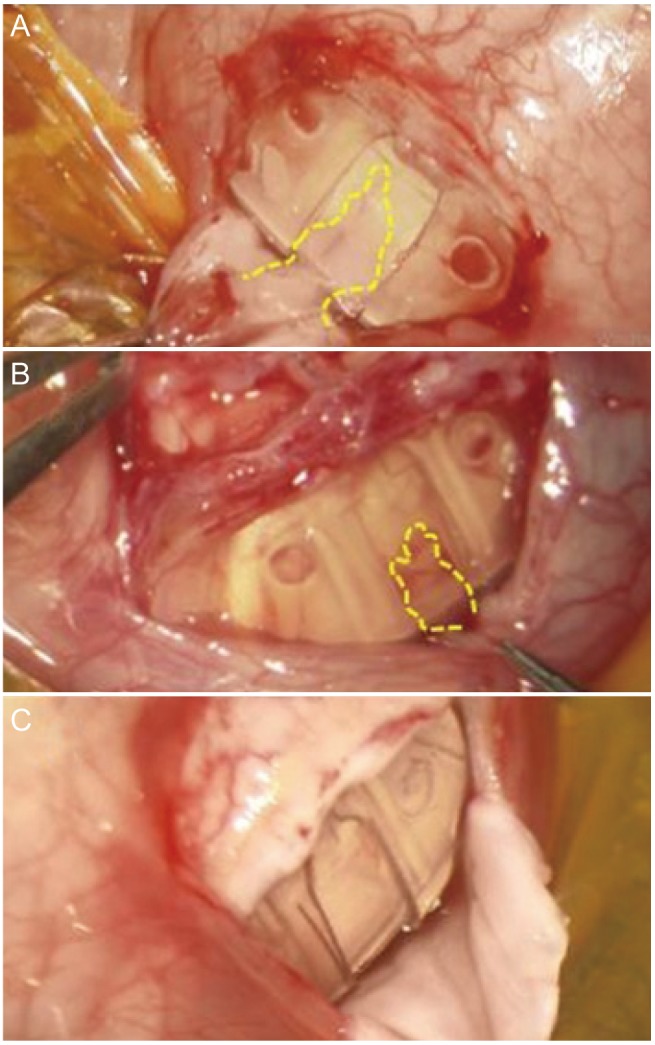
Surgery was conducted under subtenon's anesthesia. The conjunctiva was incised parallel to and 10 mm apart from the corneal limbus over the body of the AGV (
Fig. 1A). The encapsulated bleb observed under the conjunctiva was incised until the AGV body was exposed. To prevent damage to the AGV, the bleb was cut gradually using a No. 15 blade; the blade was turned upside down to use the upward-facing blade to extend the bleb incision when the aqueous humor began to seep out (
Fig. 1B, 1C). The size of the bleb excision was sufficient to expose the chamber (7 × 8 mm), which was composed of two elastic sheets (valve leaflets) connected to the tube outlet and the cover over it and extended as far posterior as possible. Then, the chamber of the AGV was checked to confirm for possible fibrovascular tissue ingrowth (
Fig. 1D). If present, it was removed by pulling the connected bleb, taking care not to cut the stalk of the fibrovascular tissue (
Fig. 1E, 1F). A 10 × 10 × 2-mm collagen matrix (Ologen, model 870051; Aeon Astron Europe BV, Leiden, The Netherlands) was inserted into the bleb excised area (
Fig. 1G, 1H), and a few anchoring sutures were placed at the anterior margin of the bleb excision, followed by continuous suturing of the conjunctiva (
Fig. 1I). After checking the stability and depth of the anterior chamber, the surgery was completed (
Fig. 1J). After surgery, 1% prednisolone acetate and 3 mg/mL ofloxacin were given four times daily. Ofloxacin was used for 4 weeks, and the duration of 1% prednisolone acetate use was adjusted depending on the status of the inflammatory reaction. At 2 weeks, 1 month, 2 months, 4 months, and 6 months after surgery, measurement of the IOP, slit lamp microscopy, and a fundus examination were performed. If postoperative IOP-lowering eye drops were considered necessary (taking into consideration any history of systemic or local adverse reactions to the drug), timolol–dorzolamide fixed-combination, brimonidine, and latanoprost were added in that order.
 | Fig. 1Encapsulated bleb excision with collagen matrix implantation. (A) Conjunctival incision 10 mm from the corneal limbus. (B) Encapsulated bleb incision. (C) The encapsulated bleb was carefully dissected. (D) Fibrovascular ingrowth into the Ahmed glaucoma valve chamber was checked. (E) The stalk of the fibrovascular tissue connecting encapsulated bleb was identified. (F) The fibrovascular tissue was removed, taking care not to cut the stalk. (G) Ologen was inserted between the Ahmed glaucoma valve and the conjunctiva. (H) Margins of both Ologen and the bleb excision were well approximated and a few anchoring sutures were performed. (I) Continuous conjunctival suture. (J) The anterior chamber was kept deep at the end of the surgery.
|
Statistical analysis was performed using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). Changes in the IOP before and after ocular massage and changes in the IOP before and after needling were analyzed. Patients were divided into two groups based on their IOP changes after ocular massage: group 1 contained those who exhibited substantial IOP reductions and group 2 contained all other patients. The Mann-Whitney U-test was used to compare IOPs and the numbers of IOP-lowering eye drops used between groups 1 and 2 after surgery. The Wilcoxon signed-rank test was used to analyze the changes in IOP, and the number of IOP-lowering eye drops after surgery in each group. A value of p ≤ 0.05 was considered statistically significant.
Go to :

Results
Of the 18 patients, 13 were male, and five were female. Their mean age was 54.6 years, ranging from 22 to 70 years. The period from primary AGV implantation to encapsulated bleb revision averaged 24.1 months, ranging from 2 to 101 months. Three patients had primary open-angle glaucoma, five had neovascular glaucoma, and ten had secondary glaucoma, including steroid-induced glaucoma and glaucoma associated with traumatic hyphema or uveitis. The best-corrected visual acuity before surgery was 0.4 ± 0.4 logarithm of the minimum angle of resolution and the mean deviation using a Humphrey Field Analyzer (Carl Zeiss Meditec, Dublin, CA, USA) was −15.7 ± 10.0 dB.
The mean preoperative IOP was 30.6 ± 5.7 mmHg, decreasing to 26.5 ± 7.7 mmHg after ocular massage (mean IOP reduction: 4.1 ± 7.6 mmHg,
p = 0.036). Two patients (group 1, ocular massage responders) showed considerable IOP reductions after ocular massage (>19.3 mmHg, mean IOP reduction + two standard deviations) with IOP reductions of 22 and 26 mmHg. In the remaining 16 patients (group 2, ocular massage non-responders), the mean IOP reduction was 1.6 ± 2.2 mmHg (
p = 0.013) (
Table 1). In group 2, additional needling was performed. The mean IOP decreased by 16.1 ± 5.2 mmHg after needling (
p < 0.001) (
Table 1).
Table 1
Preoperative IOP changes after ocular massage and needling


There was no difference in demographics, including preoperative IOP, number of IOP-lowering eye drops, and duration from primary AGV implantation to encapsulated bleb revision between groups 1 and 2 (all,
p > 0.05) (
Table 2). Fibrovascular ingrowth into the AGV was observed in 12 of 18 patients during surgery. Fibrovascular ingrowth was observed in both patients in group 1 (
Fig. 2A) and in 10 of 16 patients in group 2 (
Fig. 2B) (
p = 0.529). No fibrovascular ingrowth was observed in six of 16 patients in group 2 (
Fig. 2C).
 | Fig. 2Microscopic findings of the Ahmed glaucoma valve. (A) Thick fibrovascular ingrowth observed in ocular massage responders. (B) A relatively thin fibrovascular ingrowth observed in ocular massage non-responders. (C) No fibrovascular ingrowth was observed in ocular massage non-responders.
|
Table 2
Demographics of ocular massage responders and non-responders

In all 18 patients, postoperative IOP was significantly lower than in the preoperative period at 2 weeks, 1 month, 2 months, 4 months, and 6 months after surgery (all,
p ≤ 0.001). In both groups, IOP was lower at all postoperative time points than before surgery, but statistical significance was observed only in group 2 (
p ≤ 0.001). The IOP of group 1 was lower than that of group 2 at all postoperative time points, but statistical significance was observed only at 2 months after surgery (
p = 0.026) (
Fig. 3). The number of IOP-lowering eye drops used was lower after surgery than before surgery in all patients until 6 months (all,
p ≤ 0.011). In group 2, the number of IOP-lowering eye drops was consistently lower than before surgery until 6 months after surgery (all,
p ≤ 0.031). In group 1, there was no use of IOP-lowering eye drops until 6 months after surgery (
Fig. 4). Among the 18 patients, no additional surgery for IOP control was required until 6 months after surgery. There were no postoperative complications such as hypotony (<6 mmHg) lasting more than 1 month, choroidal effusion, or other complications. Conjunctival suturing was necessary for one patient for wound dehiscence at 1 week after surgery.
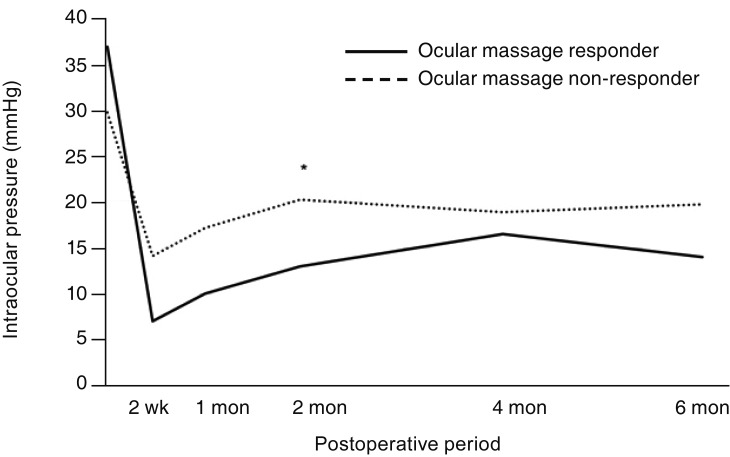 | Fig. 3The postoperative mean intraocular pressure (IOP) of patients. The postoperative time points were 2 weeks, 1 month, 2 months, 4 months, and 6 months. Although the IOP was lower in the ocular massage responders than in the ocular massage non-responders at all of the postoperative time points, the p-value of the difference was significant only at postoperative 2 months (*Mann-Whitney U-test, p = 0.026). The IOPs at all of the postoperative time points were significantly lower than preoperative IOPs in all patients (18 patients) and in ocular massage non-responders (16 patients; all, p ≤ 0.001).
|
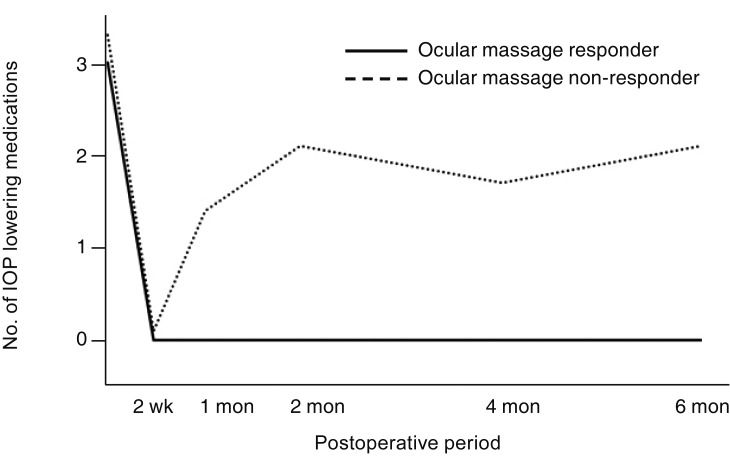 | Fig. 4The numbers of mean intraocular pressure (IOP)-lowering medications of patients. The postoperative time points were at 2 weeks, 1 month, 2 months, 4 months, and 6 months. Although the number of IOP-lowering medications used by ocular massage responders was less than that used by ocular massage non-responders at all postoperative time points, there was no significant difference between the two groups at any postoperative time point (Mann-Whitney U-test; all, p > 0.05). The numbers of IOP-lowering medications used postoperatively were significantly lower than preoperatively at all postoperative time points in all patients (18 patients; all, p ≤ 0.011) and in ocular massage non-responders (16 patients; all, p ≤ 0.031).
|
Go to :

Discussion
We performed encapsulated bleb excision using biodegradable collagen matrix implantation for failure of AGV implantation in 18 glaucoma patients whose IOP was not controlled despite the maximum tolerable medical therapy. As a result, their IOPs were significantly lowered up to 6 months postoperatively, and critical complications did not occur except for wound dehiscence in one patient. Therefore, encapsulated bleb excision with collagen matrix implantation appears to be an effective surgical treatment for failure after AGV implantation.
The AGV, one of several types of glaucoma drainage devices, is a valved device. One end of a silicone tube is inserted into the anterior chamber and the other end connected to valvular leaflets, which are integral restrictors of aqueous outflow [
1920]. The AGV is therefore less likely to develop early postoperative hypotony, choroidal effusion, and suprachoroidal hemorrhage compared to Baerveldt or Molteno implants, which are nonvalved shunts without outflow restrictors [
4142021]. However, a hypertensive phase with the IOP elevated again at 3 to 6 weeks after surgery is reported to be more common with the AGV (30% to 82%) than with nonvalved shunts, such as the Baerveldt or Molteno implants (20% to 44%) [
1415212223]. The most common cause of IOP elevation after AGV implantation is the formation of an encapsulated bleb due to excessive fibrosis around the body of the AGV during the wound-healing process [
23171924].
To date, there is no consensus regarding treatment after failure of AGV implantation because of limited research [
2111718]. Al-Mosallamy [
18] compared bleb excision (12 patients) and reimplantation of the AGV (13 patients). The failure rate of the bleb excision group was 8.3% and that of the AGV reimplantation group was 15.4%; this difference was not significant. Implant-related complications occurred only in the AGV reimplantation group. In one patient, (7.7%) the tube contacted the cornea, and two patients (15.4%) had tube erosion. Shah et al. [
17] compared surgical results between shunt revision (12 patients) and additional shunt implantation (21 patients). The mean postoperative IOP of the shunt-revision group was 25.3 mmHg, which was significantly higher than the 17.7 mmHg of the additional-tube-implantation group (
p = 0.037). The incidence of corneal edema as a postoperative complication was high in both the shunt-revision group (n = 5, 42%) and the additional-shunt-implantation group (n = 9, 43%) (
p = 1.000). Rosentreter et al. [
2] reported that 10 × 10 × 2-mm Ologen implants (Ologen ver. 2; Aeron Astron, Taipei, Taiwan), an earlier model of the Ologen used in our study, were inserted after encapsulated bleb excision in 10 out of 19 patients whose IOP was not controlled after implantation of a Baerveldt implant. The remaining nine patients received only bleb excision. Unlike our study, mitomycin C soaking was performed after bleb excision in all 19 patients. The mean follow-up durations were 11.2 and 8.6 months, respectively. At the last follow-up visit after surgery, the mean IOP of the group (17.3 mmHg) with the Ologen insertion was lower than that of the group (19.3 mmHg) without the Ologen insertion, although the difference was not statistically significant. Additional surgery to reduce IOP was not required in the group with the Ologen insertion, but three patients in the group without the Ologen insertion (33.3%) required additional surgery.
In all 18 patients included in the present study, the opening of the tube to the anterior chamber was intact, and ocular massage was performed to eliminate the possibility of temporary occlusion of the posterior part of the tube or valve leaflets. In two of 18 patients (group 1), a single gentle ocular massage induced a sudden drop in the IOP, so surgery was postponed. However, these two patients eventually underwent surgery because the IOP was repeatedly above 30 mmHg at subsequent follow-up visits. In these two patients, thick fibrovascular tissue grew into the chamber containing a valve leaflet of the AGV, which can cause the IOP to rise by preventing the valve to open. During ocular massage, the valve temporarily opened allowing the IOP to drop to normal. However, the fibrovascular tissue remained and was probably responsible for the repeated IOP increases that occurred.
Previous studies have reported fibrovascular ingrowth into AGVs, as observed in 12 of the 18 patients in the present study [
192526]. Hill et al. [
26] replaced the AGV in five patients who were suspected of having obstructions in the valves, with all five patients having fibrovascular ingrowth. They suggested that during surgery, if the device is held too firmly along the center line, a gap may form due to damage to the plastic rivet that connects the upper cover and the lower plate that covers the valve leaflets, with fibrovascular ingrowth being generated through this gap. Careful manipulation is therefore needed so as not to press on the center of the AGV during surgery. Trigler et al. [
25] reported that in six pediatric patients with a mean age of 27.5 months, failed AGVs were removed at 6 to 65 months after implantation; all contained fibrovascular ingrowth. They suggested that the incidence of fibrovascular ingrowth may be higher in children than in adults because of the increased wound-healing response in children. They concluded that fibrovascular ingrowth was the cause of all six surgical failures based on the observation that blebs did not elevate after tube irrigation during surgery. However, failure of bleb elevation by tube irrigation may be induced not only by fibrovascular ingrowth but also by an excessively thick and dense encapsulated bleb [
12]. Tube irrigation was not performed in our study, but preoperative ocular massage was performed; the degree of IOP reduction was slight in 16 patients (the IOP reduction after ocular massage was ≤6 mmHg). Needling was performed in these patients. All 16 patients, including 10 with fibrovascular ingrowth, had a smooth flow of aqueous humor from the encapsulated bleb to under the conjunctiva, and there was an additional IOP drop, which ranged from 6 to 30 mmHg. We therefore speculate that fibrovascular ingrowth was not the major cause of IOP elevation after AGV implantation in our patients. If the valve had been completely closed by fibrovascular ingrowth, then the aqueous reservoir would not have formed properly around the AGV. In our cases, even after ocular massage or needling, there was no or little flow of aqueous humor from the bleb and the IOP could not be expected to drop. Therefore, it is probable that the IOP increases in the 16 patients resulted from bleb encapsulation rather than dysfunction of the AGV. Fibrovascular ingrowth was carefully removed in the present study. There were no cases that had a shallow anterior chamber during or after surgery, and there was no persistent hypotony. Thus, removal of fibrovascular ingrowth does not appear to adversely affect the function of the valve.
Ologen was inserted in our study after excision of the encapsulated bleb. Ologen is a collagen matrix that consists of crosslinking of 90% type 1 atelocollagen and 10% glycosaminoglycan, with high biocompatibility and biodegradability, and has been reported to aid in the formation of normal tissue rather than scar tissue after surgery [
2427]. The Ologen (model 870051) used in this study has higher biocompatibility than the previous model because of lower antigenicity [
2428]. Rho et al. [
29] recently inserted Ologen together with primary AGV implantation. Compared with the AGV alone, the bleb volume in the AGV with Ologen was higher, and the incidence of the hypertensive phase was lower in the AGV with Ologen (4.5% vs. 47.6%,
p = 0.002). In our study, by using Ologen, we expected the IOP to be more effectively controlled. We expected a decreased risk of AGV exposure compared to covering only the conjunctiva over the body of the AGV after bleb excision, and also expected to reduce the recurrence of fibrovascular ingrowth by preventing direct contact of the AGV with the conjunctiva.
In conclusion, encapsulated bleb excision with collagen matrix implantation was a safe and effective surgical technique in patients with failure after AGV implantation, although we could not determine the long-term efficacy of surgical treatment because of the small number of enrolled patients and the short postoperative follow-up period. Fibrovascular ingrowth into an AGV appears to be a common finding, but it is not a major cause of failure of AGV implantation. Valve dysfunction due to fibrovascular ingrowth should be considered as a possible cause of IOP elevation after AGV implantation in patients showing sudden IOP reduction af ter ocular massage but IOP re-elevation.
Go to :











 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download