Abstract
Purpose
Croup is a common respiratory disease in children. The aim of this study was to analyze the epidemiology, etiology, and seasonal variations of respiratory virus infections in children with croup.
Methods
From October 2009 to September 2017, children admitted with croup to Gachon University Gil Medical Center under the age of 18 years were enrolled in this study. We retrospectively reviewed patients’ medical records.
Results
A total of 1,053 of 27,330 patients (3.9%) infected with lower respiratory infections were diagnosed as having croup. In the age distribution, croup was most common (50.0%) in children aged 1 to <2 years. There were 2 peaks, the major in summer (July to August) and the minor in spring (March to May). Parainfluenza virus type 1 (15.8%) was most prevalent and coincided with the summer peaks of croup. Influenza virus type B and parainfluenza virus type 3 were the most frequent etiologic agents in a spring peak of croup. Although parainfluenza virus type 1 was predominant of all ages, human coronavirus was a significant cause of croup in children younger than 1 year, whereas influenza virus played an important role in children above the age of 3 years.
References
1. Denny FW, Clyde WA Jr. Acute lower respiratory tract infections in nonhospitalized children. J Pediatr. 1986; 108(5 Pt 1):635–46.

2. Petrocheilou A, Tanou K, Kalampouka E, Malakasioti G, Giannios C, Kaditis AG. Viral croup: diagnosis and a treatment algorithm. Pediatr Pulmonol. 2014; 49:421–9.

3. Ausejo M, Saenz A, Pham B, Kellner JD, Johnson DW, Moher D, et al. The effectiveness of glucocorticoids in treating croup: metaanalysis. BMJ. 1999; 319:595–600.

4. Cherry JD. Croup (laryngitis, laryngotracheitis, spasmodic croup, laryngotracheobronchitis, bacterial tracheitis, and laryngotracheobroncho-pneumonitis). Feign RD, Cherry J, editors. Feigin and Cherry's textbook of pediatric infectious diseases. 5th ed.Philadelphia (PA): Elsevier;2004. p. 254–68.

5. Denny FW, Murphy TF, Clyde WA Jr, Collier AM, Henderson FW. Croup: an 11-year study in a pediatric practice. Pediatrics. 1983; 71:871–6.

6. Lee DR, Lee CH, Won YK, Suh DI, Roh EJ, Lee MH, et al. Clinical characteristics of children and adolescents with croup and epiglottitis who visited 146 Emergency Departments in Korea. Korean J Pediatr. 2015; 58:380–5.

7. Rosychuk RJ, Klassen TP, Voaklander DC, Senthilselvan A, Rowe BH. Seasonality patterns in croup presentations to emergency departments in Alberta, Canada: a time series analysis. Pediatr Emerg Care. 2011; 27:256–60.
8. Marx A, Török TJ, Holman RC, Clarke MJ, Anderson LJ. Pediatric hospitalizations for croup (laryngotracheobronchitis): biennial increases associated with human parainfluenza virus 1 epidemics. J Infect Dis. 1997; 176:1423–7.

10. Monto AS. Occurrence of respiratory virus: time, place and person. Pediatr Infect Dis J. 2004; 23(1 Suppl):S58–64.

11. Rihkanen H, Rönkkö E, Nieminen T, Komsi KL, Räty R, Saxen H, et al. Respiratory viruses in laryngeal croup of young children. J Pediatr. 2008; 152:661–5.

12. Segal AO, Crighton EJ, Moineddin R, Mamdani M, Upshur RE. Croup hospitalizations in Ontario: a 14-year time-series analysis. Pediatrics. 2005; 116:51–5.

13. Laurichesse H, Dedman D, Watson JM, Zambon MC. Epidemiological features of parainfluenza virus infections: laboratory surveillance in England and Wales, 1975–1997. Eur J Epidemiol. 1999; 15:475–84.
14. van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001; 7:719–24.

15. Sung JY, Lee HJ, Eun BW, Kim SH, Lee SY, Lee JY, et al. Role of human coronavirus NL63 in hospitalized children with croup. Pediatr Infect Dis J. 2010; 29:822–6.

16. van der Hoek L, Sure K, Ihorst G, Stang A, Pyrc K, Jebbink MF, et al. Croup is associated with the novel coronavirus NL63. PLoS Med. 2005; 2:e240.

17. Jeon IS, Cho WJ, Lee J, Kim HM. Epidemiology and clinical severity of the hospitalized children with viral croup. Pediatr Infect Vaccine. 2018; 25:8–16.

18. Yeo CY, Lee SU, Cho YK, Jung HY, Ma JS. A clinical study on the patients with viral croup. Chonnam Med J. 2006; 42:187–91.
19. Park YG, Shin EC, Meng KH. A statistical standard for detecting epidemic of notifiable acute communicable diseases in Korea. Korean J Epidemiol. 1997; 19:73–80.
20. Lim JS, Woo SI, Kwon HI, Baek YH, Choi YK, Hahn YS. Clinical characteristics of acute lower respiratory tract infections due to 13 respiratory viruses detected by multiplex PCR in children. Korean J Pediatr. 2010; 53:373–9.

21. Lee SJ, Shin EW, Park EY, Oh PS, Kim KN, Yoon HS, et al. Epidemiology and clincal analysis of acute viral respiratory tract infections in children (September, 1998-May, 2003). Korean J Pediatr. 2005; 48:266–75.
22. Kwon JH, Chung YH, Lee NY, Chung EH, Ahn KM, Lee SI. An epidemiological study of acute viral lower respiratory tract infections in hospitalized children from 2002 to 2006 in Seoul, Korea. Pediatr Allergy Respir Dis. 2008; 18:26–36.
24. Kahn JS. Newly discovered respiratory viruses: significance and implications. Curr Opin Pharmacol. 2007; 7:478–83.

25. Lee SJ, Lee SH, Ha EK, Sheen YH, Sung MS, Jung YH, et al. Prevalence of respiratory virus infection with regard to age, sex, and seasonality factors: a single center experience against children hospitalized during the 10 years. Allergy Asthma Respir Dis. 2017; 5:320–5.

26. Griffin S, Ellis S, Fitzgerald-Barron A, Rose J, Egger M. Nebulised steroid in the treatment of croup: a systematic review of randomised controlled trials. Br J Gen Pract. 2000; 50:135–41.
Fig. 1.
Frequency of lower respiratory infections and croup by age group. Values are presented as number. LRI, lower respiratory infection.
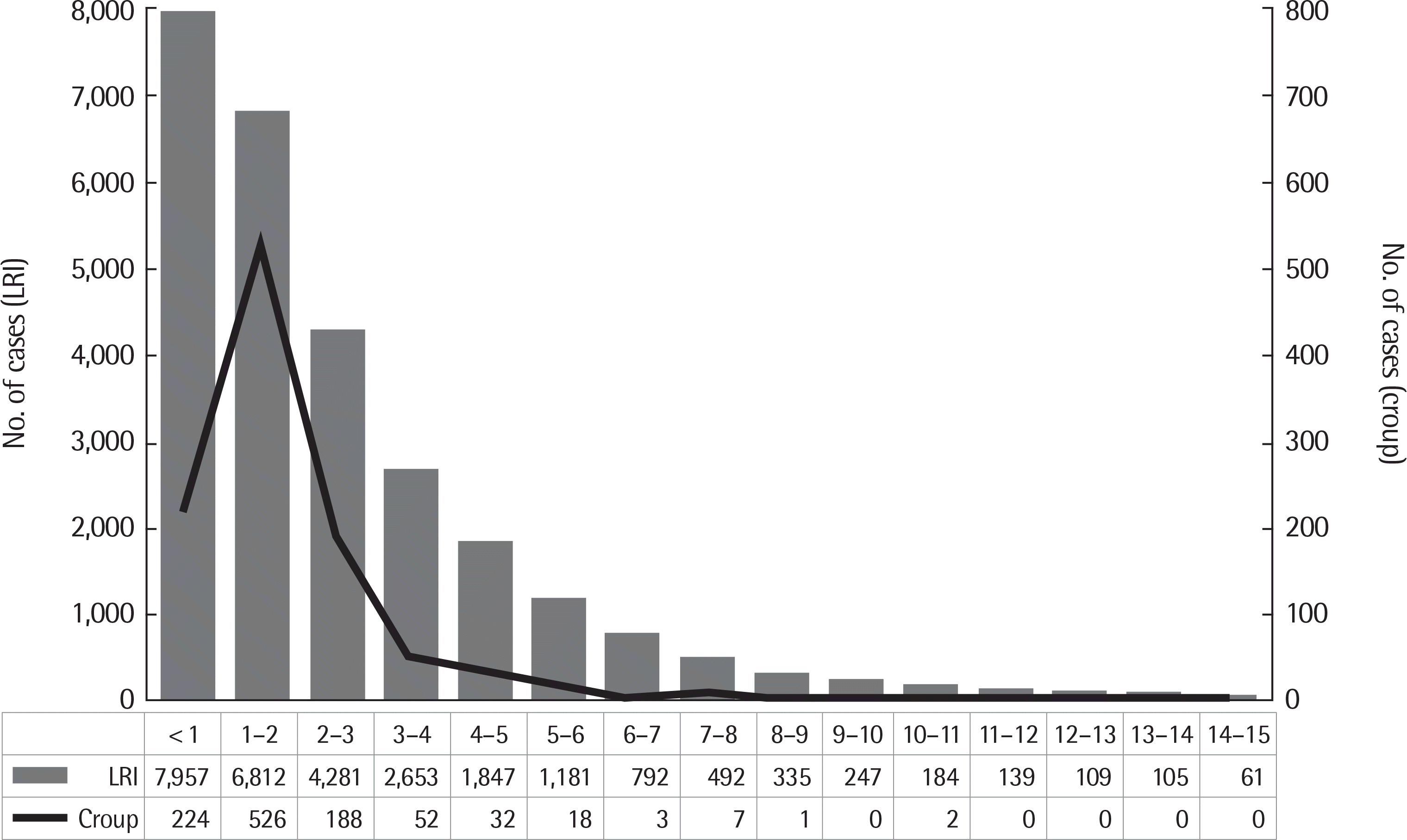
Fig. 2.
The number of viral agents in croup patients including mixed infections and simple infections. Percentage is the ratio of each virus that is mixed cases. PIV, parainfluenza virus; RSV, respiratory syncytial virus; ADV, adenovirus; IFV, influenza virus; HcoV, human coronavirus; hMPV, human metapneumovirus; HRV, human rhinovirus; HBoV, human bocavirus.
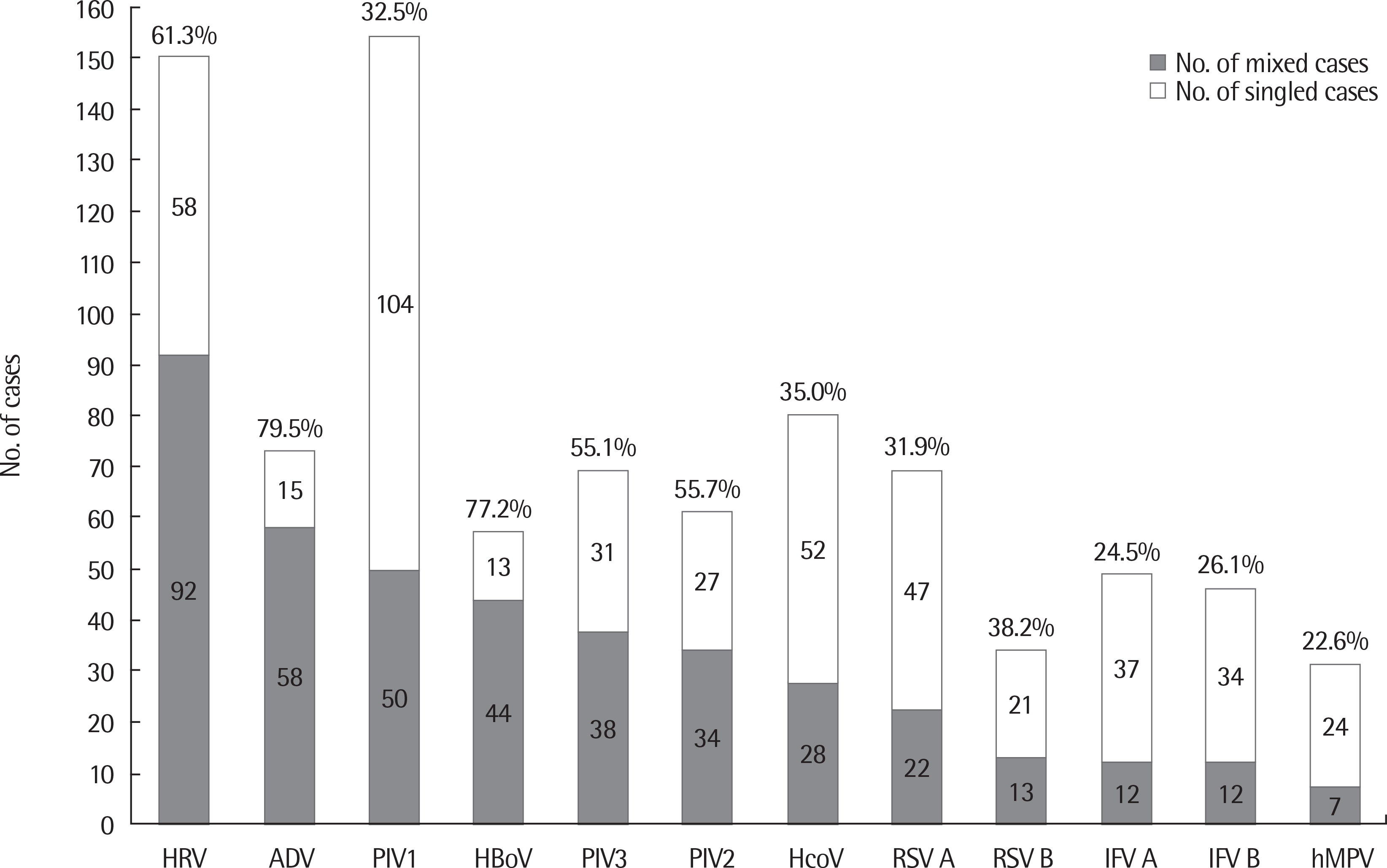
Fig. 3.
Monthly incidence of total croup and monthly incidence of croup according to 5 respiratory viruses from 2009 to 2017. PIV, parainfluenza virus; IFV, influenza virus; hMPV, human metapneumovirus.
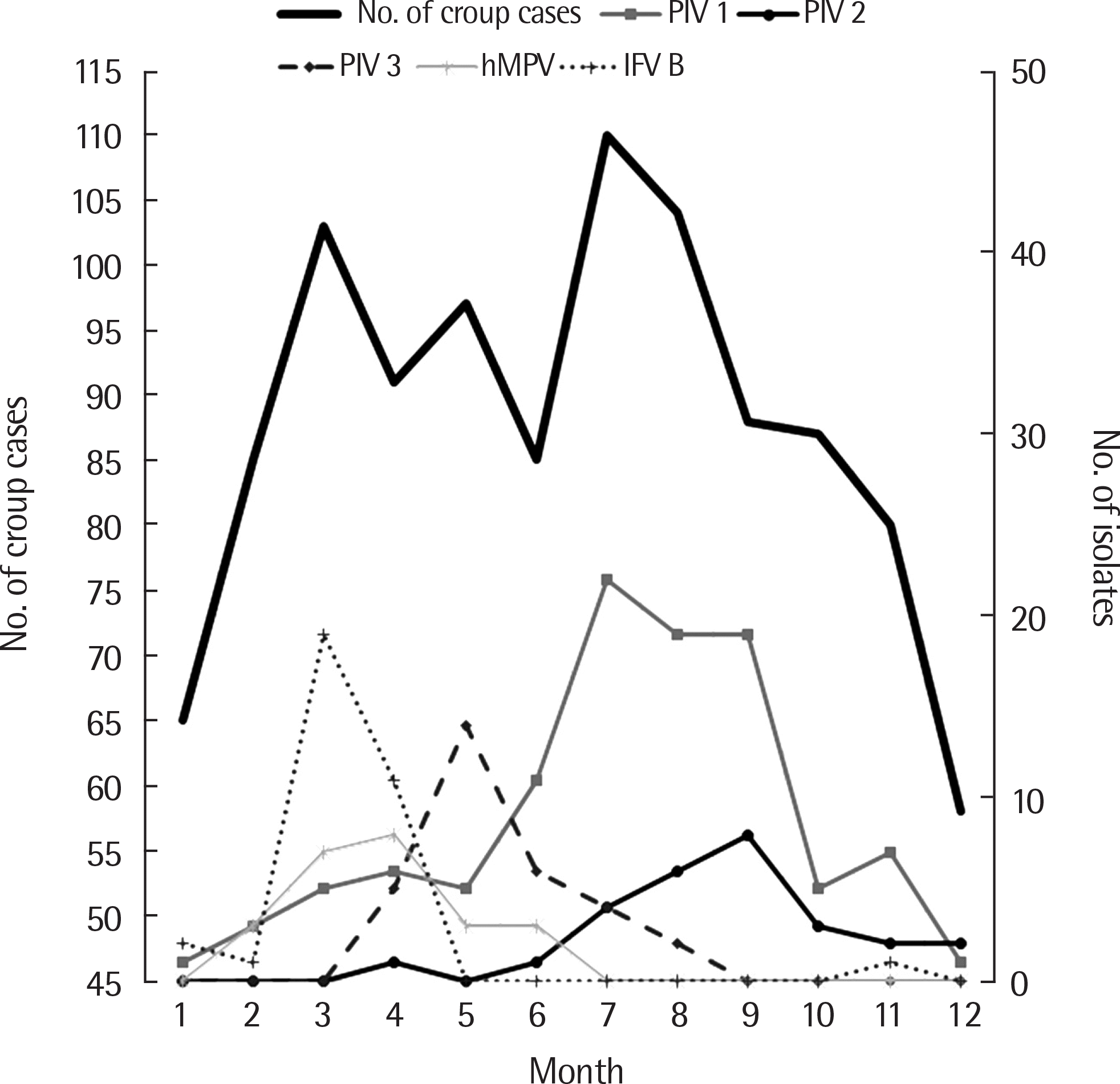
Fig. 4.
Monthly incidence of viruses detected in patients with croup from 2009 to 2017. PIV type 1,2,3,4 (A), IFV A, B and RSV A, B (B), HcoV, hMPV and HRV (C), HBoV, ADV and HEV (D). PIV, parainfluenza virus; IFV, influenza virus; RSV, respiratory syncytial virus; HcoV, human coronavirus; hMPV, human metapneumovirus; HRV, human rhinovirus; HBoV, human bocavirus; ADV, adenovirus; HEV, human enterovirus.
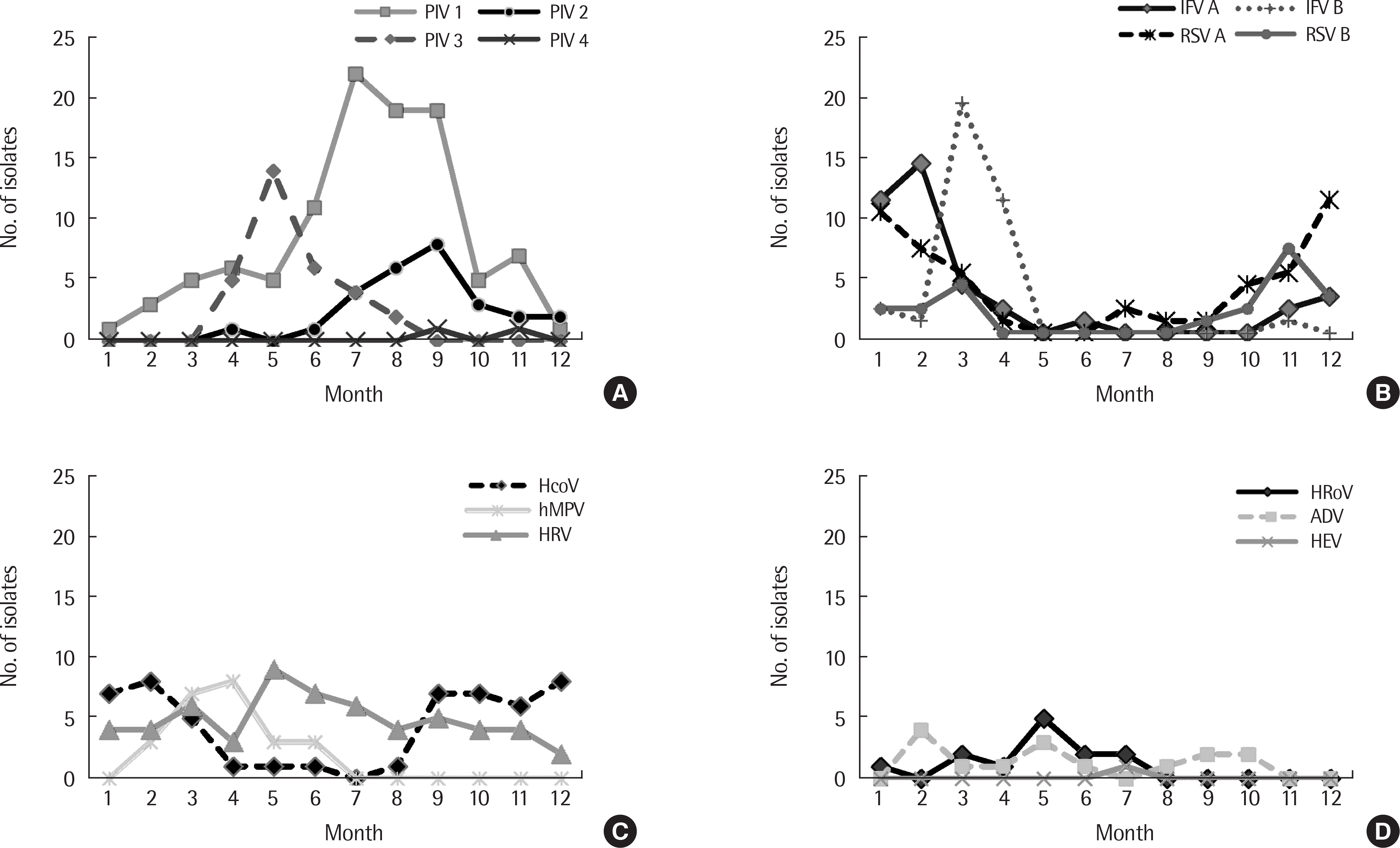
Fig. 5.
The number of cases hospitalized with croup and detected respiratory viruses per month for 8 years. The dotted arrows indicate the peaks of croup (more than 15 cases), and the solid arrows indicate viruses that affected peaks of croup. PIV, parainfluenza virus; RSV, respiratory syncytial virus; IFV, influenza virus; HcoV, human coronavirus; HRV, human rhinovirus; HBoV, human bocavirus; hMPV, human metapneumovirus.
Table 1.
Distribution and incidence by sex according to patient age in patients with croup
Table 2.
Etiologic agents isolated from patients hospitalized with croup
Table 3.
Age distribution and mean age of children hospitalized with croup for each virus
Values are presented as number (%) or mean±standard deviation. The denominator is the number of cases with isolated virus for each age group.
PIV, parainfluenza virus; RSV, respiratory syncytial virus; ADV, adenovirus; IFV, influenza virus; HcoV, human coronavirus; hMPV, human metapneumovirus; HRV, human rhinovirus; HBoV, human bocavirus.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download