Abstract
Purpose
Wet wrap therapy is a well-known treatment for severe atopic dermatitis (AD). However, wet wrap therapy with usual bandage was a troublesome and time-consuming process of application. The aim of this study was to evaluate the efficacy, safety and convenience of wet wrap therapy with new garments in children with moderate-to-severe AD.
Methods
We compared 56 AD children treated with wet wrap therapy and 14 AD children treated with only conventional therapy. We retrospectively reviewed the clinical features, change of SCORing Atopic Dermatitis (SCORAD) index, adverse effects and parent's reports.
Results
The initial mean SCORAD index was 60.3±15.3 points. No significant differences in sex, age, initial SCORAD index, total eosinophil count, total IgE level, food allergen sensitization, inhalant allergen sensitization or associated allergic diseases were found between the wet wrap and conventional groups. The pharmacological and nonpharmacological interventions except wet wrap therapy were same in the 2 groups. Wet wrap therapy with garments or tubular bandage was easily done one time per day overnight in 10.6±3.5 days by parents. Improvement in total SCORAD index, intensity, subjective symptoms and pruritus were significantly higher in the wet wrap group than in the conventional group (36.2 vs. 26.9, 6.0 vs. 4.0, 9.9 vs. 7.4, and 4.8 vs. 3.6 points). No fol-liculitis and serious adverse effects were reported.
Go to : 
References
2. Oh JW, Pyun BY, Choung JT, Ahn KM, Kim CH, Song SW, et al. Epidemiological change of atopic dermatitis and food allergy in school-aged children in Korea between 1995 and 2000. J Korean Med Sci. 2004; 19:716–23.

3. Lee Y, Choi J, Park MR, Kim J, Kim WK, Park YM, et al. Analysis of regional prevalence of allergic diseases in Korean school children. Allergy Asthma Respir Dis. 2015; 3:62–9.

4. Kim JE, Kim HJ, Lew BL, Lee KH, Hong SP, Jang YH, et al. Consensus guidelines for the treatment of atopic dermatitis in Korea (part i): general management and topical treatment. Ann Dermatol. 2015; 27:563–77.

5. Kim JE, Kim HJ, Lew BL, Lee KH, Hong SP, Jang YH, et al. Consensus guidelines for the treatment of atopic dermatitis in korea (part ii): systemic treatment. Ann Dermatol. 2015; 27:578–92.

6. Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract. 2006; 60:984–92.

7. Hon KL, Wong KY, Cheung LK, Ha G, Lam MC, Leung TF, et al. Efficacy and problems associated with using a wet-wrap garment for children with severe atopic dermatitis. J Dermatolog Treat. 2007; 18:301–5.

8. Devillers AC, Oranje AP. Efficacy and safety of ‘wet-wrap' dressings as an intervention treatment in children with severe and/or refractory atopic dermatitis: a critical review of the literature. Br J Dermatol. 2006; 154:579–85.

9. Braham SJ, Pugashetti R, Koo J, Maibach HI. Occlusive therapy in atopic dermatitis: overview. J Dermatolog Treat. 2010; 21:62–72.

10. Dabade TS, Davis DM, Wetter DA, Hand JL, McEvoy MT, Pittelkow MR, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2012; 67:100–6.

11. Oranje AP, Devillers AC, Kunz B, Jones SL, DeRaeve L, Van Gysel D, et al. Treatment of patients with atopic dermatitis using wet-wrap dressings with diluted steroids and/or emollients. An expert panel's opinion and review of the literature. J Eur Acad Dermatol Venereol. 2006; 20:1277–86.

13. Nicol NH. Atopic dermatitis: the (wet) wrap-up. Am J Nurs. 1987; 87:1560–3.
14. Goodyear HM, Spowart K, Harper JI. ‘Wet-wrap' dressings for the treatment of atopic eczema in children. Br J Dermatol. 1991; 125:604.

15. Song TW, Shin CG, Sohn MH, Kim KE. The efficacy of wet wrap therapy in children with atopic dermatitis [abstract]. Proceedings of the 2009 KAAACI-KAPARD Joint Congress; 2009 May 8–9; Seoul, Korea. Seoul: The Korean Academy of Asthma, Allergy, and Clinical Immunology;2009.
16. Janmohamed SR, Oranje AP, Devillers AC, Rizopoulos D, van Praag MC, Van Gysel D, et al. The proactive wet-wrap method with diluted corticosteroids versus emollients in children with atopic dermatitis: a prospective, randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2014; 70:1076–82.
17. Lee SJ, Lee MJ, Kim JY, Lee GS, Lee SH. Wet-wrap dressing. Korean J Dermatol. 2003; 41:1691–3.
18. Lee JH, Lee SJ, Kim D, Bang D. The effect of wet-wrap dressing on epidermal barrier in patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2007; 21:1360–8.

19. Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014; 71:116–32.
20. Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018; 32:850–78.
21. Yum HY, Kim HH, Kim HJ, Kim WK, Lee SY, Li K, et al. Current management of moderate-to-severe atopic dermatitis: a survey of allergists, pediatric allergists and dermatologists in Korea. Allergy Asthma Immunol Res. 2018; 10:253–9.

22. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980; 9292:44–7.
23. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993; 186:23–31.
24. The Korean Academy of Pediatric Allergy and Respiratory Disease. Pediatric Allergy Pulmonology & Immunology. 3rd ed.Seoul: Yeomoongak;2018. p. 202–3.
25. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63(Suppl 86):8–160.
26. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis. 1987; 136:225–44.
27. Nicol NH, Boguniewicz M, Strand M, Klinnert MD. Wet wrap therapy in children with moderate to severe atopic dermatitis in a multidisciplinary treatment program. J Allergy Clin Immunol Pract. 2014; 2:400–6.

28. Ong PY, Ferdman RM, Dunaway T, Church JA, Inderlied CB. Downregulation of atopic dermatitis-associated serum chemokines by wet-wrap treatment: a pilot study. Ann Allergy Asthma Immunol. 2008; 100:286–7.

29. González-López G, Ceballos-Rodríguez RM, González-López JJ, Feito Rodríguez M, Herranz-Pinto P. Efficacy and safety of wet wrap therapy for patients with atopic dermatitis: a systematic review and metaanalysis. Br J Dermatol. 2017; 177:688–95.

30. Hindley D, Galloway G, Murray J, Gardener L. A randomised study of “wet wraps” versus conventional treatment for atopic eczema. Arch Dis Child. 2006; 91:164–8.

31. Foelster-Holst R, Nagel F, Zoellner P, Spaeth D. Efficacy of crisis intervention treatment with topical corticosteroid prednicarbat with and without partial wet-wrap dressing in atopic dermatitis. Dermatology. 2006; 212:66–9.

32. Andersen RM, Thyssen JP, Maibach HI. The role of wet wrap therapy in skin disorders – a literature review. Acta Derm Venereol. 2015; 95:933–9.

33. Devillers AC, Oranje AP. Wet-wrap treatment in children with atopic dermatitis: a practical guideline. Pediatr Dermatol. 2012; 29:24–7.

34. Wolkerstorfer A, Visser RL, De Waard van der Spek FB, Mulder PG, Oranje AP. Efficacy and safety of wet-wrap dressings in children with severe atopic dermatitis: influence of corticosteroid dilution. Br J Dermatol. 2000; 143:999–1004.

35. Devillers AC, de Waard-van der Spek FB, Mulder PG, Oranje AP. Treatment of refractory atopic dermatitis using ‘wet-wrap' dressings and diluted corticosteroids: results of standardized treatment in both children and adults. Dermatology. 2002; 204:50–5.

Go to : 
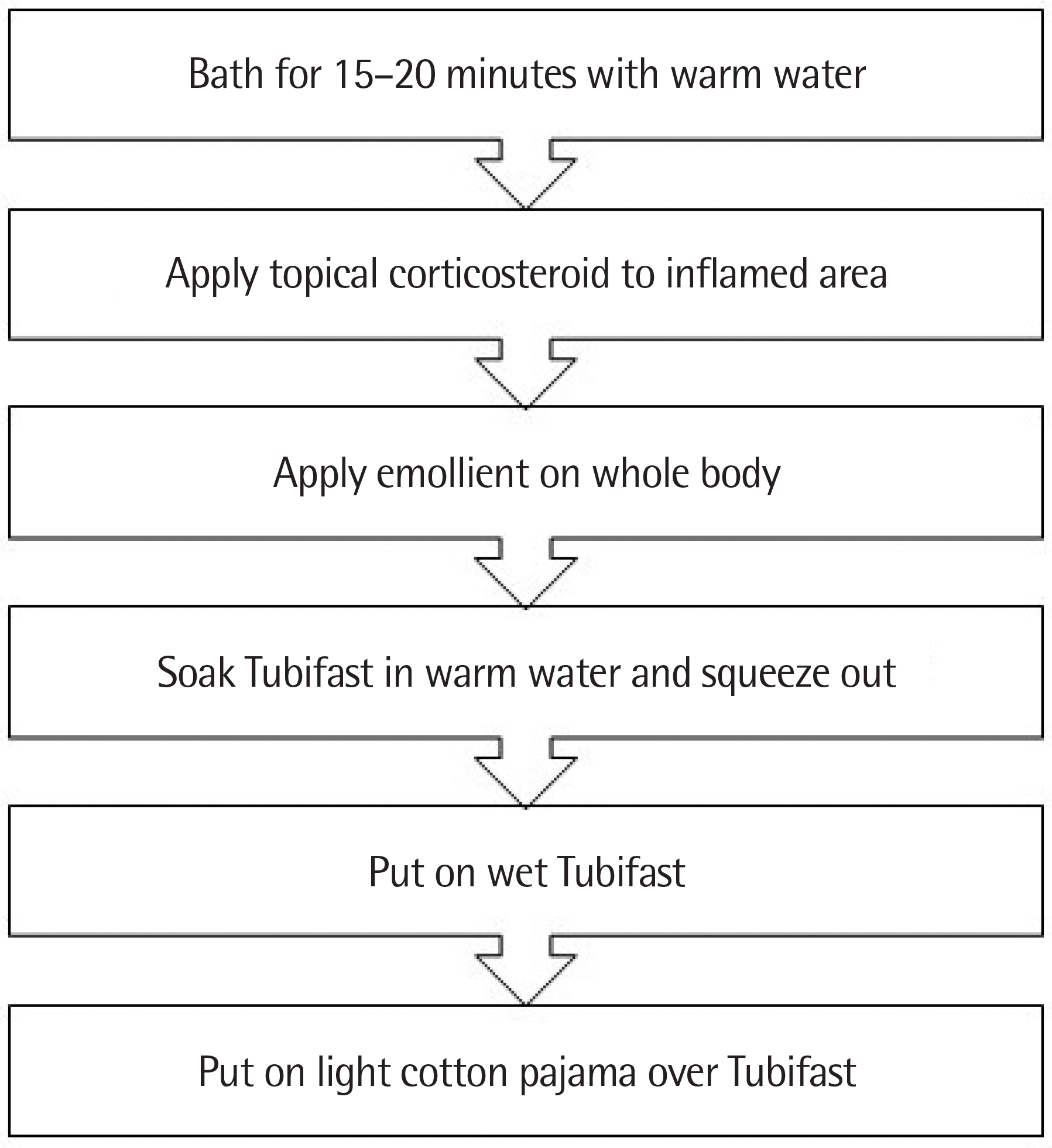 | Fig. 1.Treatment of atopic dermatitis with wet wrap therapy was done as the protocol of Inje University Ilsan Paik Hospital. |
 | Fig. 2.Tubifast garments (A), Tubifast bandage for limb (B) and/or Tubifast bandage for face (C) were applied according to the lesion of atopic dermatitis. |
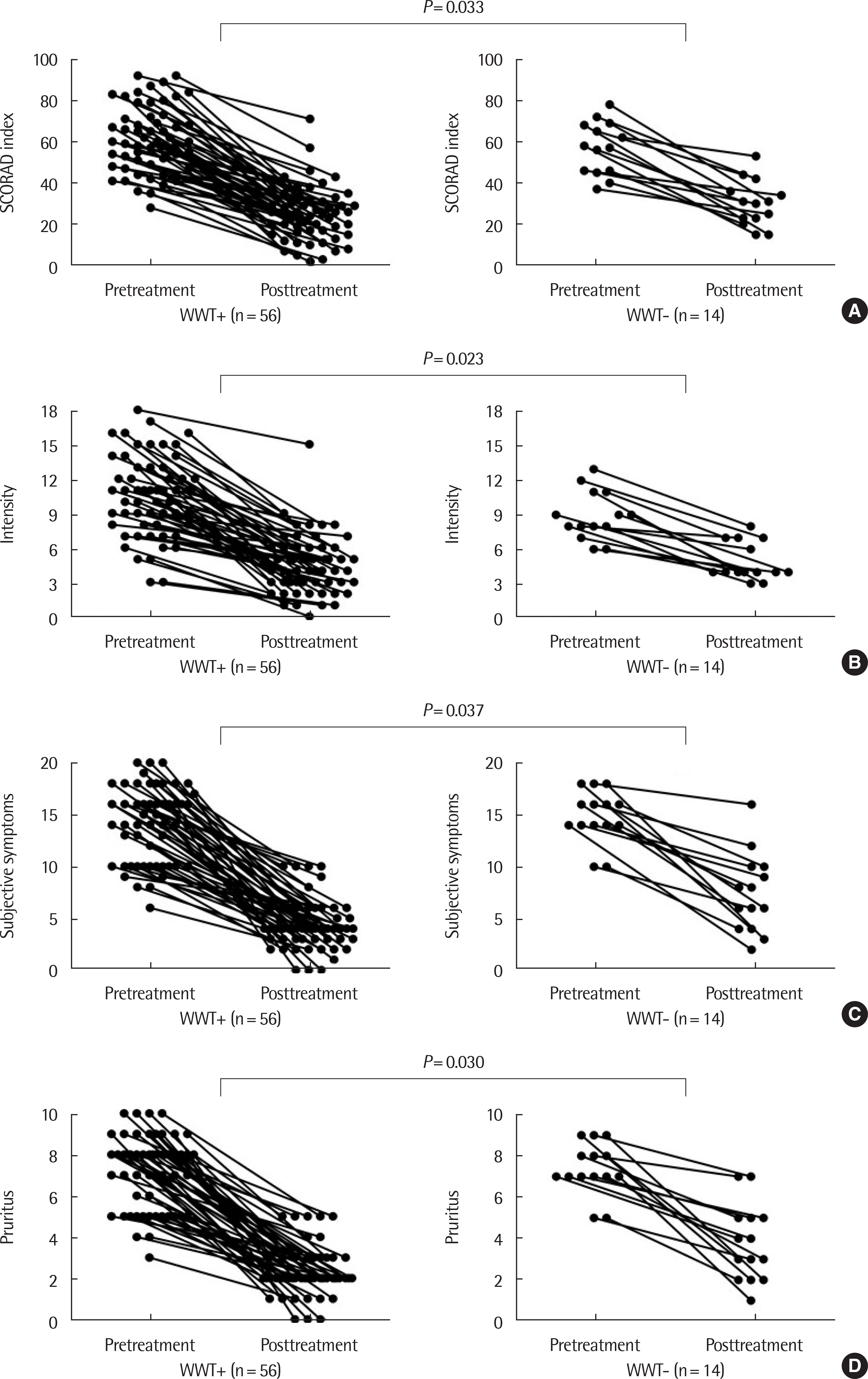 | Fig. 3.Improvement of total SCORAD index (A), intensity (B), subjective symptoms (C), and pruritus (D) were significantly higher in WWT+ group than in WWT-group (mean pretreatment score to mean posttreatment score; 61.1 to 24.9 vs. 57.1 to 30.1, 10.5 to 4.5 vs. 8.9 to 4.9, 14.2 to 4.3 vs. 14.9 to 7.4, 7.2 to 2.3 vs. 7.4 to 3.8 points). WWT+, atopic dermatitis patients with wet wrap therapy; WWT-, atopic dermatitis patients with only conventional treatment; SCORAD, SCORing Atopic Dermatitis. |
Table 1.
Comparison of clinical characteristics between WWT+ and WWT-groups
| Characteristic | WWT+ (n=56) | WWT− (n=14) | P-value |
|---|---|---|---|
| Male sex | 25 (44.6) | 10 (71.4) | 0.073* |
| Age (mo) | 45.5±47.5 | 56.1±53.1 | 0.293† |
| Initial SCORAD index | 61.1±15.9 | 57.1±12.7 | 0.436† |
| Total eosinophil count (/μL) | 890.7±753.7 | 521.4±418.3 | 0.143† |
| Total IgE (IU/mL) | 489.7±1,067.3 | 517.4±746.1 | 0.909† |
| Food allergen sensitization | 19 (33.9) | 4 (28.6) | 0.640† |
| Inhalant allergen sensitization | 16 (28.6) | 4 (28.6) | 0.959† |
| Allergic rhinitis | 13 (23.2) | 5 (35.7) | 0.338† |
| Bronchial asthma | 7 (12.5) | 0 (0) | 0.163† |
| Food allergy | 13 (23.2) | 5 (35.7) | 0.338† |
Table 2.
Comparison of improvement of outcomes between WWT+ and WWT-groups
| Outcome | WWT+ (n=56) | WWT− (n=14) | P-value |
|---|---|---|---|
| Δ SCORAD index* | 36.2±13.9 | 26.9±13.8 | 0.033 |
| Δ Objective score† | 25.7±12.4 | 19.5±10.6 | 0.097 |
| Δ Extent | 26.6±24.5 | 27.1±29.0 | 0.977 |
| Δ Intensity | 6.0±2.9 | 4.0±1.8 | 0.023 |
| Δ Subjective symptoms‡ | 9.9±3.8 | 7.4±4.1 | 0.037 |
| Δ Pruritus | 4.8±1.9 | 3.6±2.0 | 0.030 |
| Δ Sleep loss | 5.0±2.1 | 3.9±2.2 | 0.071 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download