INTRODUCTION
MATERIALS AND METHODS
German cockroach extract (GCE) preparation
ROS levels
Cell viability assay
Real-time reverse transcription polymerase chain reaction (PCR) in the A549 cells
Mouse model and treatment
Clinical evaluations and analysis in vivo
IHC
Determination of immunohistopathological staining intensity
RESULTS
GCE induces inflammation and degrades claudin-1 via a PAR2- and ROS-mediated mechanism in human bronchial epithelial cells (A549 cells)
 | Fig. 1GCE induces inflammation and degrades tight junctions in human bronchial epithelial cells (A549 cells). The 2′, 7′-DCF intensity is significantly increased following GCE exposure and suppressed following PAR2-ant and NAC treatment in A549 cells (1 hour) (A) and 2 hours (B). The expression of TSLP is significantly increased following GCE and PAR2-ago treatment and suppressed following PAR2-ant and NAC treatment in A549 cells after 2 hours (C). The expression of claudin-1 is significantly decreased by GCE and PAR2-ago treatment, but is significantly restored by PAR2-ant and NAC treatment in A549 cells (D). (A, B) Fluorescence intensity measurement at wavelengths of 450 nm and 520 nm; (C, D) real-time polymerase chain reaction (Taqman method).GCE, German cockroach extract; NAC, N-acetylcysteine; DCF, dichlorofluorescein; PAR2-ant, protease-activated receptor 2-antagonist; PAR2-ago, protease-activated receptor 2 agonist; TSLP, thymic stromal lymphopoietin.
*P < 0.01 and †P < 0.001. The experiments were repeated 6 times.
|
GCE induces allergic inflammation, which is mediated by a PAR2 and ROS mechanism, in a mouse model of asthma
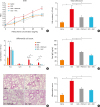 | Fig. 2GCE induces allergic inflammation in the mouse model of asthma. (A) BHR is significantly increased following GCE treatment and suppressed following PAR2-ant and NAC treatment. ‡P < 0.001 compared to saline. (B) The total number of cells in bronchoalveolar lavage fluid is also significantly increased following GCE treatment and suppressed following PAR2-ant and NAC treatment. (C) The numbers of macrophages, eosinophils and neutrophils are significantly increased following GCE treatment and suppressed following PAR2-ant and NAC treatment. (D) The total immunoglobulin E level is also significantly increased following GCE treatment and suppressed following PAR2-ant and NAC treatment. (E) Lung inflammation (hematoxylin and eosin staining 200 × magnification) and (F) peribronchial inflammation is significantly increased following GCE treatment and suppressed following PAR2 and NAC treatment.BHR, bronchial hypersensitivity; GCE, German cockroach extract; CE, cockroach extract; NAC, N-acetylcysteine; PAR2-ant, protease-activated receptor 2-antagonist.
*P < 0.05, †P < 0.01, and ‡P < 0.001. The experiments were repeated 5 times.
|
GCE induces Th2 inflammation in the lung, which is mediated by the PAR2 and ROS mechanism, in a mouse model of asthma
 | Fig. 3GCE induces T helper 2-allergic inflammation in a mouse model of asthma. The levels of IL-4 and IL-13 are significantly increased following GCE treatment and suppressed following PAR2-ant and NAC treatment in the lung (A and C). Moreover, the level of IFN-ν is significantly decreased following GCE treatment and increased following PAR2-ant and NAC treatment (B). Data were measured using real-time polymerase chain reaction.GCE, German cockroach extract; IL, interleukin; IFN, interferon; PAR2-ant, protease-activated receptor 2-antagonist; NAC, N-acetylcysteine.
*P < 0.05; †P < 0.01. The experiments were repeated 5 times.
|
GCE induces ROS-TSLP-related inflammation in the lung, which is mediated by PAR2, in a mouse model of asthma
 | Fig. 4GCE significantly suppresses GSTP1 expression in a mouse model. GSTP1 expression is significantly increased by PAR2-ant and NAC treatment in the lung, as indicated by immunohistopathology (immunohistochemistry, 200 × magnification) (A) and intensity quantification (B).GCE, German cockroach extract; GSTP1, glutathione S-transferase 1; NAC, N-acetylcysteine; PAR2-ant, protease-activated receptor 2-antagonist.
*P < 0.01; †P < 0.001. The experiments were repeated 5 times.
|
 | Fig. 5GCE significantly increases TSLP expression in a mouse model. TSLP expression is significantly suppressed by PAR2-ant and NAC treatment in the lung, as indicated by immunohistochemistry (200 × magnification) (A) and intensity quantification (B).GCE, German cockroach extract; TSLP, thymic stromal lymphopoietin; NAC, N-acetylcysteine; PAR2-ant, protease-activated receptor 2-antagonist.
*P < 0.001. The experiments were repeated 5 times.
|
GCE causes disruption of the tight junction protein in the lung, which is mediated by a PAR2- and ROS-related mechanism, in a mouse model of asthma
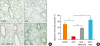 | Fig. 6GCE significantly disturbs claudin-1 expression in a mouse model. Claudin-1 expression is significantly restored by PAR2-ant and NAC treatment in the lung, as indicated by immunohistochemistry (200 × magnification) (A) and intensity quantification (B).GCE, German cockroach extract; PAR2-ant, protease-activated receptor 2-antagonist; NAC, N-acetylcysteine.
*P < 0.01; †P < 0.001. The experiments were repeated 5 times.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download