INTRODUCTION
Cold urticaria (ColdU), a subtype of chronic inducible urticaria, is characterized by the presence of itchy wheals, angioedema, or both when the skin is rewarmed after cold exposure.
1234 Although the clinical symptoms usually affect the exposed area, some individuals may have generalized wheals and systemic symptoms, including anaphylaxis in severe cases.
12 The reported annual incidence of ColdU among patients visiting dermatology clinics in Central Europe is 0.05%,
15 and its prevalence among patients with chronic urticarial in countries within the temperate zones ranges from 2.3% to 7.5%
36789; typically, the disease is found in young adult females.
1 The pathogenesis of ColdU is not yet fully understood, but it is thought to be related to mast cell degranulation and the release of histamines, as well as various inflammatory mediators.
1011 While most ColdU cases are idiopathic, some are associated with underlying diseases, such as cryoglobulinemia and infectious diseases.
1
A diagnosis of ColdU should be verified by provocation testing, which is the application of a cold stimulus (ice cubes, cool packs, cold water baths or the Temp
Test [Moxie GmbH, Berlin, Germany]) to the volar forearm of a suspected case for 5 minutes. Ten minutes after completing the test, the presence of a wheal at the test site indicates a positive result.
12 The European Academy of Allergology and Clinical Immunology/Global Allergy and Asthma European Network/European Dermatology Forum/Universidad Nacional Evangelica (EAACI/GA
2LEN/EDF/UNEV) consensus recommends the measurement of the critical stimulation time threshold (CSTT) and critical temperature threshold (CTT) to determine both disease severity and the efficacy of treatment.
1 The CSTT is the shortest duration of cold exposure which results in the presence of a wheal, whereas CTT is the highest temperature needed to stimulate a positive test reaction. A shorter CSTT or a higher CTT is indicative of a higher disease activity.
12 Currently, Temp
Test is the only validated instrument for identifying both CSTT and CTT.
12 The Temp
Test 4.0 model, a Peltier element-based temperature exposure device, provides a continuous temperature gradient from 4°C to 44°C.
12
As a result of the unclear pathogenesis of ColdU, the treatment of ColdU is based on symptomatic treatments. Previous studies have demonstrated the benefits of using sedating H
1-antihistamines (sAH
1; e.g., cyproheptadine,
1213 doxepin
13 and hydroxyzine
13) and ketotifen
12 in ColdU patients. The current EAACI/GA
2LEN/EDF/UNEV guidelines also recommend the use of a non-sAH
1 (nsAH
1) and the avoidance of cold stimuli.
1 Up-dosing of nsAH
1, omalizumab, and antibiotics (doxycycline or penicillin) have been reported to be effective in patients who are recalcitrant to the standard dose of nsAH
1.
1 Metz
et al.14 found that both 150 and 300 mg dosages of omalizumab were effective in reducing ColdU symptoms. In addition, leukotriene receptor antagonists, ciclosporin, anakinra, etanercept and ultraviolet B therapies have been reported for their effectiveness in some selected cases.
2 Cold desensitization, which is the repeated exposure to progressively colder conditions over an extended period of time, also reduces patients' symptoms. However, it should be performed under physician supervision at the beginning of treatment since it can induce anaphylaxis.
1 Regarding the disease courses, previous research demonstrates the remission rate of 18.6%–29.0%
36151617 and the mean disease duration in case of remission of 1.6–5.6 years.
3616
Even though earlier studies have provided information about the clinical courses of ColdU patients, such studies have usually been conducted in countries located in the temperate zone; hence, data related to ColdU in tropical countries are currently scarce. Thus, our study aimed to investigate the clinical features and natural course of ColdU in a tropical country.
MATERIALS AND METHODS
This was a retrospective chart review of ColdU patients aged over 18 years who visited Siriraj Urticaria Clinic, Siriraj Hospital, Bangkok, Thailand, between January 2007 and September 2018. The study protocol was approved by the Siriraj Institutional Review Board. Demographic data, clinical courses, and the results of provocation and threshold testing and other laboratory investigations were collected.
In the current study, a diagnosis of ColdU was based on the history of a patient's clinical symptoms (the appearance of wheals, angioedema or both after exposure to cold) and a confirmation via 1 or 2 provocation tests for ColdU. The instruments for the provocation tests were an ice cube; Temp
Test 4.0 and a tray filled with ice, salt and water. Patients who underwent an ice cube test had melting ice that was contained in a thin plastic bag placed on their volar forearm.
12 The Temp
Test was also conducted on the volar forearm.
12 Each test was done for 5 minutes, and the result was evaluated 10 minutes later, which allowed adequate time for the skin to rewarm.
12 The presence of a wheal at the test site after the 10 minutes indicated a positive result. Nevertheless, there were some patients with a high suspicion of ColdU despite having negative results for the ice cube test or Temp
Test. Those patients were asked to immerse one of their hands and forearms in a tray filled with ice, salt, and water for 5 minutes. This procedure was able to create a temperature as low as 1°C-3°C.The result was evaluated using the same method as for the 2 other provocation tests. In the case of a positive Temp
Test, CTT was also evaluated. For at least 1 week prior to the diagnostic tests, all patients refrained from the use of any antihistamines that they might have been using. To diagnose patients with other suspected inducible urticaria, the standard provocation testing was conducted for each type as described in a previous study.
1
As to the laboratory investigations, a retrospective review of the results was undertaken. The tests comprised a complete blood count and any additional investigations deemed necessary for individuals,
e.g., erythrocyte sedimentary rate (ESR), total serum immunoglobulin E (IgE), cryoglobulin, cryofibrinogen, cold agglutinin, antinuclear antibody (ANA), venereal disease research laboratory test, and hepatitis profiles. An autologous serum skin test was also performed as described in a previous study.
18
The assessment of the disease course was based on a patient's symptoms, which were evaluated at each follow-up visit. A remission of ColdU was defined as the absence of wheals and angioedema, despite cold exposure, and the discontinuation of any urticaria treatment for at least 6 months, as well as a negative cold-provocation test result. In the case of remissions, the disease duration was calculated from the onset of symptoms to the remission date.
Statistical analysis
Descriptive statistics (e.g., mean, minimum, maximum, standard deviation, frequency, and percentages) were utilized to describe the demographic data, disease duration, results of provocation testing and laboratory investigations. As some patients were lost to follow-up, a Kaplan-Meier survival curve was applied to determine the probability of remission at each time point. We performed the test to analyze all statistical data using SPSS Statistics for Windows, version 18 (SPSS Inc., Chicago, IL, USA).
RESULTS
Of the 1,063 chronic urticaria patients between January 2007 and September 2018, 77 (7.2%) were diagnosed with inducible urticaria. The 27 patients (2.5%) who were diagnosed with ColdU were included in this study. The clinical characteristics of those patients are summarized in
Table 1. Our patients were predominately female, and the mean age at symptom onset was 34.8 years. Other types of urticaria were concomitant with the ColdU in 37% of the patients; the most common was dermographism, followed by chronic spontaneous urticaria (CSU). A few patients had more than 2 types of urticaria. Fourteen patients (51.9%) had a personal history of atopy (mainly allergic rhinitis; n = 12, 44.4%). While most patients were healthy, there was 1 patient with chronic viral hepatitis B infection, and 2 patients had a history of cancer (breast carcinoma and cervical carcinoma). In the cases of the latter 2 patients, their cancers had been diagnosed 5 and 16 years prior to the onset of their respective ColdU symptoms, and they were both in remission (data not shown). The mean duration of individual hives occurring in daily life was 1.1 hours (range, 0.5–4.0 hours). Although two-thirds of the patients had associated angioedema, only one patient had a history of anaphylaxis. This patient had developed generalized wheals and dyspnea and had collapsed after washing clothes in cool water.
Table 1
Characteristics and natural course of cold urticaria in our 27 patients

|
Characteristics |
No. (%) |
|
Sex |
|
|
Male |
6 (22.2) |
|
Female |
21 (77.8) |
|
Mean age (yr) |
37.5 ± 15.0 (18.1, 72.1) |
|
Mean age of onset (yr) |
34.8 ± 16.5 (7.0, 71.1) |
|
Duration of hives (hr) |
1.1 ± 1.0 (0.5, 4.0) |
|
Associated other urticaria |
10 (37.0) |
|
CSU |
2 (7.4) |
|
Dermographism |
4 (14.8) |
|
CSU + dermographism |
3 (11.1) |
|
CSU + dermographism + DPU |
1 (3.7) |
|
Angioedema |
10 (37.0) |
|
History of anaphylaxis |
1 (3.7) |
|
Personal history of atopy |
14 (51.9) |
|
Atopic dermatitis |
0 |
|
Allergic rhinitis |
12 (44.4) |
|
Allergic conjunctivitis |
4 (14.8) |
|
Asthma |
4 (14.8) |
|
Treatment*
|
|
|
sAH1
|
4 (14.8) |
|
|
Cyproheptadine (1–2)†
|
3 (11.1) |
|
|
Hydroxyzine (3)†
|
1 (3.7) |
|
nsAH1
|
26 (96.3) |
|
|
Desloratadine (1–4)†
|
11 (40.7) |
|
|
Cetirizine (1–2)†
|
8 (29.6) |
|
|
Loratadine (1)†
|
4 (14.8) |
|
|
Fexofenadine (1–2)†
|
4 (14.8) |
|
|
Levocetirizine (1–4)†
|
3 (11.1) |
|
|
Bilastine (1–4)†
|
3 (11.1) |
|
Oral corticosteroids |
4 (14.8) |
|
Ciclosporin |
1 (3.7) |
|
Omalizumab |
2 (7.4) |
|
Remission (at the time of study) |
|
|
Yes |
6 (22.2) |
|
No |
21 (77.8) |
|
Mean disease duration (in 6 cases with remission) (yr) |
8.0 ± 5.6 (2.7, 17.5) |

Table 2 presents the results of our investigations on the patients. All 13 patients who underwent an ice-cube test had positive results. Temp
Test 4.0, used to evaluate 15 patients, produced 8 positive results; the mean CTT of those 8 patients was 21.0°C. With regard to the 7 patients with a negative Temp
Test result, they all subsequently achieved positive results after immersing their hands and forearms in a tray filled with ice, salt and water.
Table 2
Provocation testing, threshold testing and laboratory investigation of cold urticaria in our 27 patients

|
No. (%) |
|
Cold provocation tests*
|
|
|
Ice cube test |
|
|
|
Positive |
13/13 (100.0) |
|
TempTest
|
|
|
|
Negative |
7/15 (46.7) |
|
|
Positive |
8/15 (53.3) |
|
Immersed patient's hand and forearm in a tray filled with ice, salt and water (1°C–3°C) |
|
|
|
Positive |
7/7 (100.0) |
|
Mean CTT (°C) |
21.0 ± 3.5 (17.0, 27.0) |
|
Laboratory results |
|
|
Elevated total serum IgE |
7/8 (87.5) |
|
Positive ASST |
5/13 (38.5) |
|
Elevated ESR |
4/14 (28.6) |
|
Eosinophilia |
4/27 (14.8) |
|
Positive ANA |
2/23 (8.7) |
|
Positive HBsAg |
1/13 (7.7) |
|
Positive anti-HCV |
0/14 (0.0) |
|
Reactive VDRL test |
0/18 (0.0) |
|
Positive cryoglobulin |
0/20 (0.0) |
|
Positive cryofibrinogen |
0/15 (0.0) |
|
Positive cold agglutinin |
0/14 (0.0) |

The laboratory investigation results are detailed in
Table 2; unfortunately, since this was a retrospective study, a few items are missing. The total serum IgE was measured by nephelometry and was elevated (IgE ≥ 100 IU/mL) in 7 out of 8 patients (87.5%), with a median of 367 IU/mL and a range of 117 to 1,600 IU/mL. A positive autologous serum skin test was detected in 5 out of 13 patients (38.5%). An elevated ESR > 20 mm/hr was detected in 4 out of 14 patients (28.6%; median, 39 mm/hr; range, 24–149 mm/hr), but none of the patients with an elevated ESR had an associated infection. There was mild eosinophilia (an absolute eosinophil ≥ 500 cells/mm
3) in 4 out of 27 patients (14.8%); despite that, none were positive for parasites in a stool examination. While a positive ANA at low titer was detected in 2 out of 23 patients (8.7%), none were subsequently diagnosed with an autoimmune disease. No patient had cryoglobulinemia.
As to the treatments (
Table 1), all patients were administered sAH
1, the vast majority being an nsAH
1 (96.3%). The dosage of nsAH
1 ranged from standard up to 4-fold. In terms of the nsAH
1 drugs used, almost half of the patients received desloratadine, followed by cetirizine; the remainder were given loratadine, fexofenadine, levocetirizine or bilastine. Before the year 2014 (EAACI/GA
2LEN/EDF/UNEV guideline recommended increase dosage up to 4-fold of nsAH
1), some patients had received a combination of different types of standard dosage nsAH
1 at the same time, such as a combination of loratadine and cetirizine and a combination of loratadine and fexofenadine. In the cases of sAH
1, cyproheptadine was the most common choice, followed by hydroxyzine. A few patients were found to be recalcitrant to the sAH
1 and required other medications, such as oral corticosteroids (14.8%), ciclosporin (3.7%), and omalizumab (7.4%). Six patients (22.2%) were in remission at the time of the study, with a mean disease duration of 8.0 years (
Table 1). According to the Kaplan-Meier survival analysis (
Figure), none of the patients were in remission during the first 2 years of the disease. However, after 5 years and 10 years from the onset of symptoms, 13.8% and 42.6% of the ColdU patients were in remission, respectively. After onset, the median duration before half of the patients were in remission was 12 years.
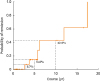 | Figure
A Kaplan-Meier survival analysis (one minus survival function) demonstrating the duration of ColdU (n = 27). None of the patients were in remission during the first 2 years of the disease. However, after 3, 5 and 10 years from the onset of symptoms, 6.7%, 13.8%, and 42.6% of ColdU patients, respectively, were in remission. The median duration, when 50% of patients were in remission, was 12 years.
ColdU, cold urticaria.

|
DISCUSSION
The prevalence of ColdU among chronic urticaria patients in our tropical country is currently 2.5%. Our group previously reported a prevalence of ColdU among chronic urticaria patients of 1.8% and 1.7% in the years 2007 and 2011, respectively.
1719 By comparison, the reported prevalences of ColdU among chronic urticaria patients in countries located in the temperate zones have ranged from 2.3% to 7.5%.
36789 All studies, including this present one, have found that the majority of ColdU patients are females.
36151620 On the other hand, while the mean age at symptom onset in our study was 34.8 years, the means reported by those conducted in the temperate zones were slightly to moderately lower (17.8–32.5 years).
361620 Of the 27 cases of ColdU in the current study, 37% of the patients were associated with other types of urticaria, the large majority being dermographism and CSU. One patient had a combination of ColdU, CSU, dermographism, and delayed pressure urticaria. Details of the research carried out in countries in temperate climates are given in
Table 3, and the ColdU patients in those studies were concomitant with dermographism, cholinergic urticaria, heat urticaria, and CSU.
61516
Table 3
Comparison of clinical features and natural course of ColdU reported by our study and previous studies*

|
Author (yr) |
City (country) |
Latitude |
Climate zone |
Average temperature in winter (°C) |
Prevalence of ColdU among all chronic urticaria cases (%) |
No. (%) |
Female (%) |
Mean age at symptom onset (yr) |
Atopy (%) |
History of anaphylaxis (%) |
Concomitant form of other urticaria |
Mean CTT (°C) |
Mean disease duration† (yr) |
|
This study (2018) |
Bangkok (Thailand) |
13.7°N |
Tropical |
27.9 |
2.5 |
27 |
77.8 |
34.8 |
51.9 |
3.7 |
- CSU 7.4% |
21.0 |
8.0 |
|
- Dermographism 14.8% |
|
- CSU + dermographism 11.1% |
|
- CSU + dermographism + DPU 3.7% |
|
Deza et al.,3 (2016) |
Barcelona (Spain) |
41.4°N |
Temperate |
10.3 |
7.2 |
74 |
63.5 |
32.0 |
33.8 |
18.9 |
NA |
14.0 |
5.3 |
|
Jain et al.,15 (2016) |
Australian Capital Territory (Australia) |
35.5°S |
Temperate |
12.7 |
NA |
99 |
62.6 |
22.0‡
|
46.5 |
28.3 |
- Dermographism 16.2% |
NA |
2.8§
|
|
- Cholinergic 16.2% |
|
- Heat 8.1% |
|
- CSU 6.1% |
|
- DPU 4.0% |
|
- Vibratory 1.0% |
|
Katsarou-Katsari et al.,6 (2008) |
Athens (Greece) |
38.0°N |
Temperate |
7.8 |
2.3 |
62 |
51.6 |
32.5 |
33.9 |
29.0 |
- Dermographism 12.9% |
NA |
5.6 |
|
- Cholinergic 12.9% |
|
- Dermographism + cholinergic 3.2% |
|
Wanderer et al.,20 (1986) |
Denver (USA) |
39.5°N |
Temperate |
0.2 |
NA |
50 |
54.0 |
17.8 |
22.0 |
38.0 |
NA |
NA |
NA |
|
San Diego (USA) |
32.7°N |
Temperate |
15.9 |
|
Neittaanmäki,16 (1985) |
Helsinki, Kuopio, Gulu, and Turku (Finland) |
60.2°N |
Temperate |
−5.2 |
NA |
220 |
63.0 |
25.1 |
55.0 |
- Fainting/shock 7.2% |
- Dermographism 19.1% |
NA |
1.6 |
|
- Dyspnea 14.1% |
- Cholinergic 6.8% |
|
- CSU 1.8% |
|
- Heat urticaria 0.9% |
|
- CSU + dermographism 0.9% |
|
- Cholinergic + dermographism 0.9% |

Atopy, thought to be involved in an IgE-dependent pathogenesis for ColdU,
1415 was detected in half of our patients (14 out of 27; 51.9%). Allergic rhinitis, asthma, and allergic conjunctivitis were reported in 12 patients (44.4%), 4 patients (14.8%) and 4 patients (14.8%), respectively. That result was similar to the 1995 findings of Visitsunthorn
et al.,
12 namely, that 3 out of 6 ColdU children (50%) in Bangkok, Thailand, had atopic conditions (1 out of 6 had allergic rhinitis, and 2 out of 6 were diagnosed with asthma). In contrast, research conducted in temperate countries reported that various proportions of ColdU patients (ranging from 22.0% to 55.0%) were associated with atopy (
Table 3).
36151620 Vichyanond
et al.
21 reported that the prevalence rates of allergic rhinitis and asthma in healthy subjects in 1997 and 1998 in Bangkok, Thailand, were 26.3% and 8.8%, respectively. This implies that the prevalence of atopy was higher in our ColdU patients than in the healthy population. Nonetheless, the study by Neittaanmäki
16 in Finland found that the prevalence of atopy in ColdU patients was nearly the same as in the normal population.
One patient (3.7%) in the present study had a history of anaphylaxis. This contrasted with the findings of studies conducted within temperate countries, namely, that 18.9%–38.0% of the patients experienced a history of anaphylaxis and the study of Neittaanmäki
16 in Finland revealed the prevalence of faint/shock and dyspnea of 7.2% and 14.1%, respectively (
Table 3).
361520 The current study was conducted at Siriraj Hospital, which is a tertiary care center located in Bangkok, and most of the hospital's patients are from Bangkok and its environs. Since the highest CTT of ColdU in our study was 27.0°C and the mean temperature during winter in Bangkok is 27.9°C, patients' bodies are only infrequently exposed to a temperature below their own CTT. Consequently, anaphylaxis caused by ColdU might be detected less often in a tropical country.
In order to diagnose ColdU in patients whose CTT was less than 4°C, the patient's hand and forearm were immersed in a tray filled with ice, salt, and water, which was able to create a temperature of 1°C–3°C for 5 minutes.
22 All 7 patients who had previously had a negative Temp
Test result obtained a positive result after performing this immersion test. As 20.0%–28.4% of the ColdU patients in previous studies had negative results after doing an ice cube test, those patients were diagnosed as atypical ColdU.
320 It could be that their CTT might have been less than 4°C. Thus, immersing a patient's hand and forearm in a tray filled with ice, salt and water might improve the sensitivity of cold provocation testing in patients with a negative ice cube test or Temp
Test result.
The laboratory abnormalities detected in our patients were elevated levels of total serum IgE and ESR, eosinophilia, and positive ANA and hepatitis B surface antigen. The interaction of IgE antibodies with a cold-dependent skin antigen has been hypothesized as the pathogenesis of ColdU.
4 Seven out of 8 ColdU patients (87.5%) who had a blood test for total serum IgE in this study showed an increased level of total serum IgE. This correlated with the increased prevalence of atopy in the current study. Further investigation should be undertaken in a larger population. Even though elevated ESR was detected in 4 out of 14 patients in our study, this condition was non-specific, and no-one had an associated infection. One patient with chronic viral hepatitis B infection (an inactive hepatitis B virus carrier), which had been diagnosed 2 years before the onset of her urticaria symptoms, had normal ESR and liver function testing and did not receive any medication for this condition. Even though previous studies have demonstrated that most ColdU cases are idiopathic, some cases have been associated with underlying diseases, including cryoglobulinemia,
120 hematologic malignancy,
20 leukocytoclastic vasculitis,
120 and infectious diseases. In comparison, none of our patients had secondary causes of ColdU.
Prior to the EAACI/GA
2LEN/EDF/UNEV recommendation of increase dosage up to 4-fold of nsAH
1, experts, for example Grattan
et al.,
23 suggested that at least 2 nsAH
1 at the same time might be offered to patients who did not response to single standard dosage nsAH
1. Therefore, some patients in our study had received a combination of different types of nsAH
1 at the same time according to the retrospective nature of our study. Later on, the current EAACI/GA
2LEN/EDF/UNEV recommendations for ColdU management are to utilize an nsAH
1, for its treatment and to avoid triggering factors.
12 Moreover, the use of up to 4-fold dosages of the nsAH
1, the addition of ciclosporin or omalizumab, and cold desensitization have proven effective in recalcitrant cases.
1 Some studies have suggested that sAH
1 and corticosteroids are also beneficial in the treatment of ColdU.
11 In our study, all patients were treated with sAH
1 (mostly nsAH
1), but a few (14.8%) needed an intermittent short course of prednisolone (5–20 mg/day), ciclosporin (3 mg/kg/day) or omalizumab (150–300 mg every 4 weeks) to control their disease symptoms.
The Kaplan-Meier survival analysis demonstrated that 13.8% and 42.6% of patients in the present study had a remission within the first 5 and 10 years, respectively. Other studies have reported a wide range of 5-year (17.9%–26.6%) and 10-year remission rates (24.5%–44.5%).
3615
As our ColdU prevalence was only 2.5% out of 1,063 chronic urticaria patients, only 27 ColdU patients were included in this study. In addition, since this was a retrospective study, some data were missing.
In summary, the rate of anaphylaxis in patients with ColdU in a tropical country was lower than the reported levels in temperate countries. Furthermore, the number of female patients, mean age at symptom onset, atopy rate, rate of concomitant CSU and mean CTT were higher than the corresponding data reported for ColdU patients in temperate countries. On the other hand, the remission rate and the rate of our patients whose CTT was less than 4°C were similar to those found by studies conducted in temperate countries.








 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download