INTRODUCTION
MATERIALS AND METHODS
Study design and patients
Study endpoints
Statistical analysis
RESULTS
Patients
 | Fig. 2SIROCCO trial design: Korean patients.*Q4W, every 4 weeks; Q8W, every 8 weeks (first three doses Q4W).
*Unless indicated, values presented are for all patients regardless of blood eosinophil counts.
|
Table 1
Patient demographics and baseline clinical characteristics

Efficacy
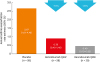 | Fig. 3Effect of benralizumab on annual asthma exacerbation rates for Korean patients with baseline blood eosinophil counts ≥ 300 cells/μL receiving high-dosage ICS/LABA.CI, confidence interval; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; Q4W, every 4 weeks; Q8W, every 8 weeks (first three doses Q4W).
*Estimates calculated via a negative binomial model with adjustment for treatment, oral corticosteroid use, and prior exacerbations.
|
Table 2
Summary of efficacy results for Korean patients in SIROCCO receiving high-dosage ICS/LABA with blood eosinophil counts ≥ 300 cells/μL

 | Fig. 4Effect of benralizumab on prebronchodilator FEV1 for Korean patients with baseline blood eosinophil counts ≥ 300 cells/µL receiving high-dosage ICS/LABA.BD, bronchodilator; CI, confidence interval; FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; Q4W, every 4 weeks; Q8W, every 8 weeks (first three doses Q4W).
*Estimates calculated using a mixed-effects model for repeated measures analysis, with adjustment for treatment, baseline value, oral corticosteroid use at time of randomization, visit, and visit × treatment.
|
 | Fig. 5Effect of benralizumab on total asthma symptom score for Korean patients with baseline blood eosinophil counts ≥ 300 cells/μL receiving high-dosage ICS/LABA.CI, confidence interval; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; Q4W, every 4 weeks; Q8W, every 8 weeks (first three doses Q4W).
*Estimates calculated using a mixed-effects model for repeated measures analysis, with adjustment for treatment, baseline value, oral corticosteroid use at time of randomization, visit, and visit × treatment; †Scored 0–6; decreasing score indicates improvement in symptoms.
|
Safety
Table 3
Summary of adverse events (safety analysis set)

DISCUSSION
Table 4
Summary of efficacy results for patients receiving high-dosage ICS/LABA with baseline blood eosinophil counts ≥ 300 cells/μL in the Korean and overall SIROCCO trial populations (benralizumab vs. placebo)

| Korean patients in SIROCCO* | SIROCCO overall patient population15 | |||
|---|---|---|---|---|
| Benralizumab Q4W (n = 28) | Benralizumab Q8W (n = 30) | Benralizumab Q4W (n = 275) | Benralizumab Q8W (n = 267) | |
| Reduction in annual exacerbation rate (%) | ↓70‡ | ↓85† | ↓45† | ↓51† |
| Increase in prebronchodilator FEV1 (L) | ↑0.270§ | ↑0.362‡ | ↑0.106§ | ↑0.159† |
| Change in total asthma symptom score | ↓0.28 | ↑0.07 | ↓0.08 | ↓0.25§ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download