Abstract
Completion pancreatectomy (CP), is an effective, and potentially curative option for selected patients with local recurrence of pancreatic neoplasms in the remnant pancreas after initial pancreatoduodenectomy (PD). Traditionally CP has been performed via the open approach. Reports of minimally-invasive CP particularly after previous open PD are rare. We present a case of a 72-year old male who previously underwent open PD 5 years ago for intraductal papillary mucinous neoplasm (IPMN) with high grade dysplasia in the uncinate process. He had multifocal IPMN and low-risk lesions in the body and tail were managed conservatively. On routine surveillance, the cyst in the body was noted to be increasing in size with the development of a non-enhancing solid component confirmed on magnetic resonance imaging and subsequent endoscopic ultrasonography. The patient underwent successful robotic assisted laparoscopic completion pancreatectomy. Final histology confirmed a recurrent IPMN with low-to-intermediate grade dysplasia. The postoperative recovery was uneventful and he was discharged on postoperative day 9.
Today, completion pancreatectomy (CP) remains a relatively rare procedure which is technically demanding to perform and is associated with a high morbidity and mortality rate.1 The most common indications for CP include its use as a salvage procedure for patients who have experienced severe complications from postoperative pancreatic fistula after pancreaticoduodenectomy (PD) or for patients who have developed a local recurrence of pancreatic neoplasm after previous PD.234
Over the past decade, the application of minimally invasive surgery for pancreatectomies has rapidly expanded with improvements in surgical technique and surgical equipment.567 Presently, laparoscopic or robotic distal pancreatectomies has become the surgical approach of choice in many expert pancreatic centers world-wide due to its advantages over the conventional open approach such as shorter length of stay, earlier convalescence and decreased wound infection rate.678 Minimally-invasive DP has become widely accepted as opposed to PD as it is less technically demanding and does not require the surgeon to perform multiple complex surgical anastomoses.8 Not surprisingly, the adoption of minimally-invasive PD (MIPD) remains more limited today and is only routinely performed by a few surgeons practicing at specialized high-volume centers.910
Presently, there are very limited reports in the literature on the use of laparoscopy or robotic surgery for CP particularly after open PD.2 This is likely due to rarity of CP performed as an elective procedure as isolated local recurrence of pancreatic neoplasms are a rare occurrence. Furthermore, due to the technical complexity of the procedure, most of these operations have been performed via the traditional open approach. In this study, we report a case of robotic assisted laparoscopic CP performed for recurrent IPMN.
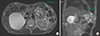
The patient is 72-year old male who underwent open PD with pancreaticogastrostomy reconstruction 5 years ago for an intraductal papillary mucinous neoplasm (IPMN) with high risk features involving the uncinate process. He also had a low risk IPMN in the body and tail which were managed conservatively. Final histology revealed IPMN with high grade dysplasia. The patient had an uneventful postoperative recovery and had regular follow-up at 6 months. During surveillance scan, the IPMN in the remnant pancreas was found to have increased in size. A non-enhancing solid component with and a worrisome feature of a thickened cyst wall had developed (Fig. 1). This was confirmed on endoscopic ultrasonography (EUS) to be an enhancing <5 mm solid component. The patient subsequently underwent robotic- assisted laparoscopic CP.
A 12-mm sub-umbilical access was used initially for camera insertion and pneumoperitoneum was established. One 12 mm working, one 5-mm trocar in the left paraumbilical, 8 mm left flank, 8 mm epigastric, and 8 mm right hypochondrium ports were placed. Diagnostic laparoscopy was initially performed to determine the extent of adhesion. Laparoscopic dissection was subsequently performed using the Harmonic scalpel. Numerous adhesions were divided, followed by entrance into the lesser sac via the gastrocolic ligament and short gastric vessels. The pancreatic body and tail were dissected and mobilized from the spleen to the stomach. The spleen was completely mobilized and detached from its attachments. After meticulous mobilization of the pancreatic remnant, pancreaticogastrostomy was identified at the posterior wall of the stomach. The splenic artery and vein were also identified, clipped and divided. Finally, the pancreaticogastrostomy anastomosis was then resected with a cuff of the posterior wall of the stomach and the specimen bagged. Da Vinci Si robot (Intuitive Surgical) was then docked and the posterior gastrostomy was closed with V-loc and vicryl 3/0. A single drain was placed along the stomach bed and left sub-diaphragmatic area. The total operating time was 460 min. Intraoperative blood loss was 300 mL without blood transfusion.
The postoperative course was uneventful and the patient was discharged well on postoperative day 9. The final pathological finding was compatible with recurrent IPMN with low-to-intermediate grade dysplasia. There were 14 benign lymph nodes and the final resection margins were clear. The patient remained well on review at 6 months postoperatively.
CP, when technically feasible, is a safe, effective, and potentially curative option in selected patients with isolated local recurrence of pancreatic neoplasms in the remnant pancreas after initial pancreatectomy.111213 Classically, CP has been performed via the conventional open approach.111213 Over the past decade, there has been a rapid increase in the adoption of laparoscopic and robotic pancreatic surgery with numerous studies demonstrating its superiority especially in terms of shorter hospital stay and quicker convalescence over its traditional open counterpart.6789 Presently, both laparoscopic and robotic approaches have been reported for the various types of pancreatectomies including DP, PD, central pancreatectomies and enucleations.56714 More recently, 2 single-center randomized controlled trials have confirmed the superior short-term outcomes of laparoscopic PD over its open counterpart.910
Nonetheless, the minimally-invasive approach for CP has been rarely reported and to the best of our knowledge only 2 studies215 reporting on 3 cases have been published to date (Table 1). This is likely due to the combination of: 1) the rarity for the need of CP especially in the elective setting as isolated local recurrence of pancreatic neoplasms are rare and 2) the perceived technical difficulty of the procedure due to the frequent presence of dense adhesions occurring after PD. Sunagawa et al reported the first laparoscopic CP after previous LPD in 2013.15 The patient developed remnant pancreatic cancer eighteen months after LPD for distal bile duct cancer. LCP was performed successfully and the patient was discharged without complications on postoperative day 15. Subsequently, Sahakyan et al.2 reported 2 cases LCP after OPD in 2016. The first case was performed for pancreatic cancer in the remnant pancreas after previous OPD for ampullary cancer. The patient recovered uneventfully without complications. The 2nd case was performed for recurrent metastases in the remnant pancreas from renal cell carcinoma after previous OPD. The patient had to undergo reoperation for bleeding on postoperative day 1. However, the patient subsequently recovered well.
In the present case, to the best of our knowledge, we describe the first case of robotic-assisted laparoscopic CP reported in the English literature. We elected to perform a diagnostic laparoscopy initially to determine the extent of adhesions and feasibility of the minimally invasive approach. Subsequently, lysis of adhesions and dissection of the pancreas was continued laparoscopically via a combination of blunt and sharp dissection. In our opinion the laparoscopic approach may be superior to the robotic approach for surgical dissection in this case as dense adhesions and distorted surgical anatomy frequently required a combination of sharp and blunt dissection whereby the presence of tactile feedback was important. The robot was docked for the final part of the procedure to close the posterior gastrostomy. In this case due to the presence of adhesions and previous gastrojejunostomy, the stomach could not be rotated and the posterior gastrostomy had to be closed “upside down” in a tight narrow space. The superior dexterity of the robotic endo-wrists compared to conventional laparoscopy allowed us to perform this procedure easily.
In conclusion, we demonstrated the feasibility and safety of robotic assisted laparoscopic CP after open PD. This study demonstrates that the minimally-invasive approach can be considered in selected patients allowing these patients to benefit from the MIS approach. Further studies with a larger patient cohort are needed to confirm these findings.
Figures and Tables
ACKNOWLEDGEMENTS
Dr Brian Goh has received travel grants and proctor fees from Transmedic, the local distributor of Da Vinci robot in Singapore.
References
1. Bressan AK, Wahba M, Dixon E, Ball CG. Completion pancreatectomy in the acute management of pancreatic fistula after pancreaticoduodenectomy: a systematic review and qualitative synthesis of the literature. HPB (Oxford). 2018; 20:20–27.

2. Sahakyan MA, Yaqub S, Kazaryan AM, Villanger O, Berstad AE, Labori KJ, et al. Laparoscopic completion pancreatectomy for local recurrence in the pancreatic remnant after pancreaticoduodenectomy: case reports and review of the literature. J Gastrointest Cancer. 2016; 47:509–513.

3. Kleeff J, Reiser C, Hinz U, Bachmann J, Debus J, Jaeger D, et al. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann Surg. 2007; 245:566–572.

4. Miura F, Takada T, Amano H, Yoshida M, Isaka T, Toyota N, et al. Repeated pancreatectomy after pancreatoduodenectomy. J Gastrointest Surg. 2007; 11:179–186.

5. Goh BKP, Low TY, Lee SY, Chan CY, Chung AYF, Ooi LLPJ. Initial experience with robotic pancreatic surgery in Singapore: single institution experience with 30 consecutive cases. ANZ J Surg. 2019; 89:206–210.

6. Goh BKP, Lee SY, Kam JH, Soh HL, Cheow PC, Chow PKH, et al. Evolution of minimally invasive distal pancreatectomies at a single institution. J Minim Access Surg. 2018; 14:140–145.

7. Caba Molina D, Lambreton F, Arrangoiz Majul R. Trends in robotic pancreaticoduodenectomy and distal pancreatectomy. J Laparoendosc Adv Surg Tech A. 2019; 29:147–151.

8. Joechle K, Conrad C. Cost-effectiveness of minimally invasive pancreatic resection. J Hepatobiliary Pancreat Sci. 2018; 25:291–298.

9. Palanivelu C, Senthilnathan P, Sabnis SC, Babu NS, Srivatsan Gurumurthy S, Anand Vijai N, et al. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br J Surg. 2017; 104:1443–1450.

10. Poves I, Burdío F, Morató O, Iglesias M, Radosevic A, Ilzarbe L, et al. Comparison of perioperative outcomes between laparoscopic and open approach for pancreatoduodenectomy: the PADULAP randomized controlled trial. Ann Surg. 2018; 268:731–739.
11. Koizumi M, Sata N, Kasahara N, Morishima K, Sasanuma H, Sakuma Y, et al. Remnant pancreatectomy for recurrent or metachronous pancreatic carcinoma detected by FDG-PET: two case reports. JOP. 2010; 11:36–40.
12. Strobel O, Hartwig W, Hackert T, Hinz U, Berens V, Grenacher L, et al. Re-resection for isolated local recurrence of pancreatic cancer is feasible, safe, and associated with encouraging survival. Ann Surg Oncol. 2013; 20:964–972.

13. Miyazaki M, Yoshitomi H, Shimizu H, Ohtsuka M, Yoshidome H, Furukawa K, et al. Repeat pancreatectomy for pancreatic ductal cancer recurrence in the remnant pancreas after initial pancreatectomy: is it worthwhile? Surgery. 2014; 155:58–66.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download