Abstract
Portal vein (PV) size matching between recipient and liver graft is important in preventing anastomotic stenosis in living donor liver transplantation (LDLT). In right liver grafts, the diameter of graft PV is usually >10 mm. Thus, PV size matching does not become critical in adult recipients. If the recipient PV is very large, funneling fence can be attached to graft PV. However, if the diameter of graft PV is <8 mm, it can induce anastomotic stenosis. We experienced a few cases of PV anastomotic stenosis due to small-sized graft PV in >5000 LDLT cases, but graft PV widening was not performed because graft PV is considered as being a no-touch area. In thinking out of the box, we performed wedged-patch venoplasty to exceptionally narrow graft PV. A 4 year-old female patient underwent second LDLT due to progressive deterioration of graft function after 3 years. At first LDLT operation for biliary stresia, an iliac vein conduit was interposed for PV reconstruction. At second LDLT operation, the diameter of interposed PV was 10 mm, but the left liver graft PV was only 6 mm-sized. Uniquely, the left PV was waist only at first-order PV. To resolve this PV waist, a longitudinal incision was made to release the waist. A cold-preserved fresh iliac vein patch was inserted to widen the PV orifice. The patch size was adjusted to match the size of the recipient PV. The patient recovered uneventfully. This wedged-patch venoplasty technique can be applied to small-sized graft PV, to cope with PV size mismatching in LDLT.
Size matching of the portal vein (PV) between the recipient and liver graft is important in preventing anastomotic stenosis in living donor liver transplantation (LDLT). In LDLT using a right liver graft, the diameter of right liver graft PV is usually >10 mm, thus PV size matching does not become a critical problem in adult recipients. If the recipient PV is small, unification venoplasty of two PV braches can be applied.1 If the recipient PV is very large, a funneling fence can be attached to the graft PV for easy anastomosis.2 However, if the diameter of graft PV is <8 mm, it can induce anastomotic stenosis even though a growth factor is fully given at the suture material. We have experienced a few cases of PV anastomotic stenosis so far due to small-sized graft PV in >5000 LDLT cases, but graft PV widening was not performed because graft PV is considered as being a no-touch area.
In thinking out of the box, we performed wedged-patch venoplasty to an exceptionally narrow graft PV to cope with PV size mismatching in LDLT. We herein present the case and describe the surgical technique of wedged-patch venoplasty.
A 14 kg-weighing 4 year-old female patient underwent second LDLT due to progressive deterioration of graft function for 3 years (Fig. 1). The first LDLT operation was performed due to biliary stresia at age of 11 months and the donor was her mother. At the second LDLT, the donor was the 35 year-old sister of her mother and a 350 g-weighing whole left liver with middle hepatic vein trunk was harvested. The left liver graft PV was only 6 mm, because the left PV was waisted at the first-order PV (Fig. 2).
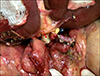
In contrast, the diameter of the recipient PV was 12 mm because an iliac vein conduit was interposed to cope with portal hypoplasia at the first LDLT operation (Fig. 1). To resolve this graft PV waist-associated size mismatching, a longitudinal incision was made at the graft PV stump to release the waist. A small cold-preserved fresh iliac vein patch was attached to widen the graft PV orifice (Fig. 3). The size of patch was adjusted to match with the size of the recipient PV.
In addition to the PV venoplasty, the left and middle hepatic vein orifices were unified, and a vein patch was attached to the middle hepatic vein side to widen the conjoined outflow orifice according to our standardized LDLT procedures (Fig. 4).
Graft implantation was uneventful, along the orders of hepatic vein anastomosis under total occlusion of the inferior vena cava, end-to-end portal vein anastomosis, portal reperfusion, hepatic artery anastomosis under surgical microscopy and hepaticojejunostomy using the previously made jejunal limb (Fig. 5). For hepatic vein and PV reconstruction, we used continuous running sutures using 5-0 and 6-0 polydioxanone (PDS) respectively. We have do not applied interrupted sutures combined with continuous running sutures using polypropylene (Prolene).
The patient recovered uneventfully and is doing well to date for 6 months. The portal vein showed a streamlined configuration without anastomotic stenosis after the second LDLT operation (Fig. 6).
In pediatric LDLT, PV stenosis is one of the most common and most critical complications. Patients with biliary atresia have suffered from periportal inflammation and fibrosis due to recurrent cholangitis, by which the recipient PV becomes hypoplastic and sclerotic PV.34 To cope with such an intractable PV anatomy, we adopted an interposition graft for PV reconstruction.5 The favorable long-term result of PV interposition graft was shown at the time of retransplantation operation in this case.
In this case, graft PV was smaller than the recipient PV. Such size mismatching of PV is often encountered in adult LDLT because of aneurysmal dilatation of recipient PV. In most cases of adult LDLT using a right liver graft, direct anastomosis of PVs is often feasible despite presence of considerable size discrepancy. If the recipient PV is too large to perform direct anastomosis, a funneling fence can be attached to the graft PV for easy anastomosis.2 However, if the diameter of right liver graft PV is <8 mm, it can induce anastomotic stenosis even though a growth factor is fully given at the suture material. We have experienced a few cases of PV anastomotic stenosis so far due to small-sized graft PV in >5000 LDLT cases, but graft PV widening was not performed because graft PV is considered as being a no-touch area.
In donor liver anatomy of this case, the native left PV has a waist at its first-order level, which is an unusual finding. Since manipulation of the single graft PV is usually unnecessary, we have considered it as a no-touch area. Meanwhile, unification of two graft PV orifices is a standard procedure.678910 In thinking out of the box, we performed wedged-patch venoplasty to exceptionally narrow graft PV. The surgical technique for wedged-patch venoplasty is intuitive and simple because we accumulated similar experience on graft hepatic vein venoplasty, as shown in this case.11 The lesson learned from this case can be applied to adult LDLT cases which has an unusually small graft PV.
In conclusion, we believe that this wedged-patch venoplasty technique can be applied to small-sized graft PV to cope with PV size mismatching in LDLT.
Figures and Tables
Fig. 1
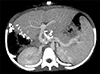
Pretransplant computed tomography findings of the recipient. The first liver graft was severely damaged (A), but the interposed portal vein was well maintained (B).
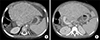
Fig. 2
Computed tomography findings of the donor. The left liver was small (A) and a waist is present at the first-order left portal vein (B, arrow).
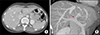
Fig. 3
Operative photographs of portal vein (PV) venoplasty. The ventral wall of the graft PV is incised and a large-sized patch is attached (A). A vein patch is anastomosed (B). The final shape of enlarged graft PV is visible (C), and its diameter is more than 15 mm (D).
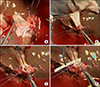
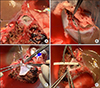
Fig. 4
Operative photographs of hepatic vein (HV) venoplasty. The middle and left HVs are unified (A and B). An incision is applied at the middle HV trunk and a vein patch is attached (C). The size of conjoined outflow orifice was measured to be approximately 30 mm (D).
References
1. Kang SH, Hwang S, Jung DH, Ahn CS, Moon DB, Ha TY, et al. Unification venoplasty to cope with recipient portal vein anomaly during living donor liver transplantation. Transplant Proc. 2013; 45:3000–3004.

2. Moon DB, Lee SG, Ahn CS, Ha TY, Park GC, Yu YD. Side-to-end renoportal anastomosis using an externally stented polytetrafluoroethylene vascular graft for a patient with a phlebosclerotic portal vein and a large spontaneous splenorenal shunt. J Am Coll Surg. 2011; 212:e7–e11.

3. Chen CL, Concejero A, Wang CC, Wang SH, Lin CC, Liu YW, et al. Living donor liver transplantation for biliary atresia: a single-center experience with first 100 cases. Am J Transplant. 2006; 6:2672–2679.

4. Ou HY, Concejero AM, Huang TL, Chen TY, Tsang LL, Chen CL, et al. Portal vein thrombosis in biliary atresia patients after living donor liver transplantation. Surgery. 2011; 149:40–47.

5. Hwang S, Kim DY, Ahn CS, Moon DB, Kim KM, Park GC, et al. Computational simulation-based vessel interposition reconstruction technique for portal vein hypoplasia in pediatric liver transplantation. Transplant Proc. 2013; 45:255–258.

6. Kwon JH, Hwang S, Song GW, Moon DB, Park GC, Kim SH, et al. Conjoined unification venoplasty for triple portal vein branches of right liver graft: a case report and technical refinement. Korean J Hepatobiliary Pancreat Surg. 2016; 20:61–65.

7. Lee HJ, Hwang S, Ahn CS, Kim KH, Moon DB, Ha TY, et al. Long-term outcomes of portal Y-graft interposition for anomalous right portal veins in living donor liver transplantation. Transplant Proc. 2012; 44:454–456.

8. Hwang S, Lee SG, Ahn CS, Kim KH, Moon DB, Ha TY, et al. Technique and outcome of autologous portal Y-graft interposition for anomalous right portal veins in living donor liver transplantation. Liver Transpl. 2009; 15:427–434.

9. Lee SG, Hwang S, Kim KH, Ahn CS, Park KM, Lee YJ, et al. Approach to anatomic variations of the graft portal vein in right lobe living-donor liver transplantation. Transplantation. 2003; 75:3 Suppl. S28–S32.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download