Abstract
BACKGROUND/OBJECTIVES
MATERIALS/METHODS
RESULTS
CONCLUSIONS
Figures and Tables
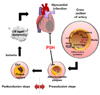 | Fig. 1Summary of development of myocardial infarction (Modified from [4]).In the preocclusion steps, LDL enters the intima due to endothelial dysfunction. LDL absorbed is oxidized to oxLDL, and oxLDL is engulfed by the macrophages. The macrophages are then transformed to foam cells which subsequently proliferate, resulting in the formation of atherosclerotic plaques and narrowing of the arteries. In the post-occlusion steps, abrupt rupture of the plaques leads to clot formation in the lesion. This event can occlude the artery and subsequently result in myocardial ischemia. As a result, ATP generation is greatly reduced due to interruption of oxidative phosphorylation, and myocardial cells die through apoptosis and necrosis. As regions of cell death become extensive, myocardial infarction ensues. PSH, psyllium seed husk; LDL, low-density lipoprotein; oxLDL, oxidized LDL.
|
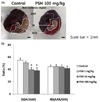 | Fig. 2Effect of PSH supplementation on infarct size.Rats underwent 30 min ischemia through ligation of LAD, followed by 3 h reperfusion through release of the ligation. (A) Evans blue dye was infused into the heart after LAD was re-ligated. The heart was harvested and cut into four slices. The slices were stained with TTC. AAR, IA, and BZA were determined as the area without infiltration of Evans blue dye, the area without TTC stain, and the area equivalent to (AAR-IA), respectively. (B) IS, the ratio of IA to AAR, and RS, the ratio of AAR to LVA, are presented. In the PSH-treated groups, PSH (1, 10, or 100 mg/kg/d) supplements were fed for 3 days prior to LAD ligation. In the control group, no PSH was administered prior to LAD ligation. The number of rats used in the control and PSH-treated (1, 10, or 100 mg/kg/d) groups were 6 per group. Values are expressed as means ± SEM. *P < 0.05, when compared to control group. PSH, psyllium seed husk; LAD, left anterior descending coronary artery; TTC, 2,3,5-triphenyltetrazolium chloride; AAR, area at risk; IA, infarct area; BZA, border zone area; IS, infarct size; RS, risk size; LVA; left ventricular area.
|
 | Fig. 3Effect of PSH supplementation on the formation of caspase-3 (CASP3) and expression of BCL-2 and BAX.(A) Western blots of CASP3 (cleaved caspase-3 generated from procaspase-3), BCL-2, and BAX in the AAR. Protein levels were measured by Western blotting for the sham, control, and PSH-treated (100 mg/kg/d) groups. ERK1 was used as the loading control. (B) Quantitative analysis of CASP3 (cleaved caspase-3), BCL-2, and BAX. C-CASP3 (CASP3)/ERK1 and BCL-2/BAX ratios are presented. The ratios were calculated by setting the control group value (0 mg/kg/d of PSH) at 1. The number of rats used in the sham, control and PSH-treated (100 mg/kg/d) groups were 6 per group. Values are expressed as means ± SEM. CASP3, caspase-3; C-CASP3, cleaved caspase-3; PSH, psyllium seed husk; AAR, area at risk.
|
 | Fig. 4Effect of PSH supplementation on the expression of NRF2, GSTM2, and SOD2.Western blots of NRF2, GSTM2, and SOD2 in the AAR are presented. (A) Protein levels were measured by Western blotting for the sham, control, and PSH-treated (100 mg/kg/d) groups. ERK1 was used as a loading control. (B) Quantitative analysis of NRF2, GSTM2, and SOD2: NRF2/ERK1, GSTM2/ERK1, and SOD2/ERK1 ratios are presented. The ratios were calculated by setting the control group value (0 mg/kg/d of PSH) at 1. The number of rats used in the sham, control and PSH-treated (100 mg/kg/d) groups were 6 per group. Values are expressed as means ± SEM. ***P < 0.001, and *P < 0.05 vs. control group. PSH, psyllium seed husk; NRF2, nuclear factor erythroid 2-related factor 2; GSTM2, glutathione S-transferase mu 2; SOD2, superoxide dismutase 2; AAR, area at risk.
|
 | Fig. 5Effect of PSH supplementation on the expression of SIRT (1–7).Western blots of SIRT (1–7) in the AAR are presented. (A) Protein levels were measured by Western blotting for the sham, control, and PSH-treated (100 mg/kg/d) groups. ERK1 was used as the loading control. (B) Quantitative analysis of SIRT (1–7): SIRT (1–7)/ERK1 ratios are presented. The ratios were calculated by setting the control group value (0 mg/kg/d of PSH) at 1. The number of rats used in the sham, control and PSH-treated (100 mg/kg/d) groups were 6 per group. Values are expressed as means ± SEM. **P < 0.01 and *P < 0.05 vs. control group. PSH, psyllium seed husk; SIRT, sirtuins; AAR, area at risk.
|
 | Fig. 6Effect of PSH supplementation on the expression of PKAβ, pCREB, and CREB.Western blots of PKAβ, pCREB, and CREB in the AAR are presented. (A) Protein levels were measured by Western blotting for the sham, control, and PSH-treated (100 mg/kg/d) groups. ERK1 was used as the loading control. (B) Quantitative analysis of PKAβ/ERK1, pCREB/ERK1, and CREB/ERK1 ratios are presented. The ratios were calculated by setting the control group value (0 mg/kg/d of PSH) at 1. The number of rats used in the sham, control and PSH-treated (100 mg/kg/d) groups were 6 per group. Values are expressed as means ± SEM. **P < 0.01 and *P < 0.05 vs. control group. PSH, psyllium seed husk; PKAβ, protein kinase Aβ; pCREB, phosphorylated cAMP response element-binding protein; AAR, area at risk.
|
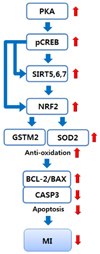 | Fig. 7A proposed, underlying mechanism for the myocardial protection through PSH supplementation.PSH supplementation protects against myocardial I/R injury through upregulation of PKA expression, CREB phosphorylation, sirtuin expression, NRF2 expression, expression of antioxidant enzymes such as GSTM2 and SOD2, and subsequent reduction of ROS toxicity, followed by reduction of apoptosis through increase of BCL-2/BAX ratio and subsequent reduction of CASP3 generation. PKA, protein kinase A; pCREB, phosphorylated cAMP response element-binding protein; SIRT5, 6, 7, sirtuins 5, 6, 7; NRF2, nuclear factor erythroid 2-related factor 2; GSTM2, glutathione S-transferase mu 2; SOD2, superoxide dismutase 2; CASP3, caspase-3; MI, myocardial infarction; PSH, psyllium seed husk; I/R, ischemia/reperfusion; ROS, reactive oxygen species.
|
Table 1
Ingredient composition of experimental diets

1)Modified AIN-93G diet was purchased in a pre-mix form.
2)One kg of basal diet was prepared by adding 50 g of corn starch to 950 g of the modified AIN-93G diet.
3)One kg of PSH diets was prepared by adding 50 g of mixture of corn starch and psyllium seed husk (PSH) to 950 g of the modified AIN-93G diet.
4)PSH diets of 1, 10 or 100 mg/kg/d refer to the corresponding dosage of PSH, given per kilogram of rat per day.
Notes
This work was supported by a grant of Comprehensive and Integrative Medicine R&D project through Comprehensive and Integrative Medicine Institute (CIMI), funded by the Ministry of Health & Welfare (MOHW), Republic of Korea (Grant Number : CIMI-15-02-08), the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2017R1D1A1B03034255) to J. L., and supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government(MSIT) (No. NRF-2017R1C1B2010220) to S. H. L.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download