The Bactrian camel is a unique animal that has well adapted to living in the Gobi Desert and semi-desert grasslands. Due to the extreme conditions of its living environment, the Bactrian camel has acquired many peculiar biological characteristics that help it survive in arid, hot, cold, windy, and sandy weather. It can subsist on a diet comprised of high-salt-content plants that are rich in cellulose but have moderate protein contents [
1]. Remarkably, Bactrian camels can also eat certain poisonous plants such as
Cynanchum and Chinese Stellera (
i.e.,
Stellera) without ill effects. The dietary characteristics of the Bactrian camel indicate the uniqueness of its metabolism and ability to deal with xenobiotics. These properties are closely related to the cytochrome P450 (CYP) system of the Bactrian camel as this system is responsible for the metabolism of most endogenous and exogenous substances in animals [
23].
CYP enzymes are a multigene family of enzymes that have a critical role in the metabolism of many drugs and xenobiotics, such as industrial chemicals, environmental pollutants, and carcinogens [
4]. The CYP1A enzyme participates in the degradation and metabolism of many drugs, and its activity can be enhanced or decreased by certain agents. P-acetaminophen is metabolized mainly by the CYP1A enzyme to produce sulfate and glucuronic acid conjugates. It is a commonly used probe substrate for the CYP1A enzyme [
5] because the activity of the enzyme can be determined by measuring the dynamic blood concentration of p-acetaminophen and/or its metabolites. The
in vitro activity of the CYP1A enzyme from the Bactrian camel has been reported [
6]; however, there are no reported studies on the
in vivo activity of this enzyme in the Bactrian camel. In this study, the pharmacokinetic properties of p-acetaminophen were studied in Bactrian camel in order to ascertain its ability to metabolize drugs and determine its CYP1A enzyme activity
in vivo. The results provide a scientific basis for the clinical use of p-acetaminophen and related substances.
All study procedures and animal care activities were conducted in accordance with the Bioethics Committee of the College of Veterinary Medicine at Inner Mongolia Agricultural University (12150000460029509N). Twelve six-year-old lactating Bactrian camels were randomly assigned to group 1 (administered substrate only) and group 2 (administered substrate plus enzyme inhibitor) with 6 camels in each group. Camels in group 1 were intramuscularly injected in the neck with a single dose of p-acetaminophen (4 mg/kg). Camels in group 2 were given lomefloxacin hydrochloride (0.4 mg/kg) by intramuscular injection via the same route once a day for 4 successive days, and 2 h after the last injection each camel received a single dose of p-acetaminophen (4 mg/kg) intramuscularly. All experimental camels were from West Alxa, Inner Mongolia, and China. None of the experimental camels had received any drugs for at least 6 months prior to this study. All subjects were fasted for 12 h overnight before each experiment and were not allowed food, but water was provided ad libitum throughout the study.
Blood samples (4 mL) were collected from the jugular vein in heparinized tubes at 0, 0.083, 0.25, 0.5, 1, 2, 4, 6, 12, 24, 36, and 48 h following administration of p-acetaminophen. The plasma concentrations of p-acetaminophen were determined by high-performance liquid chromatography using ultraviolet (UV) detection. A Supelco Discovery C18 column (250 mm × 4.6 mm, 5 μm; Sigma-Aldrich, USA) was used for separation. The mobile phase consisted of 20% methanol and 80% water, and the flow rate was 0.5 mL/min. The UV detection wavelength was 248 nm, and the sample injection volume was 8 μL. The 2-acetaminophen internal standard (1 mg) was weighed precisely and dissolved in the mobile phase (methanol/water = 20/80) to prepare a 100 µg/mL internal standard solution. Similarly, 1 mg of standard p-acetaminophen was weighed precisely and dissolved in the mobile phase to prepare a 100 µg/mL p-acetaminophen standard solution.
The pharmacokinetic parameters for p-acetaminophen were calculated using the noncompartmental approach as implemented in commercially available pharmacokinetics software (Phoenix WinNonlin, Version 7.0; Pharsight Corporation, USA). All data are expressed as mean ± standard deviation values, while the plasma concentration-time data are presented in semi-logarithmic plots. Single factor analysis of variance was used to validate the experimental design. Graph Pad Prism 5 (GraphPad Software, USA) was used to test the significance of the parameter differences between the 2 groups.
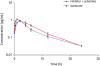
The main pharmacokinetic parameter values for p-acetaminophen in Bactrian camels in the 2 study groups and the plasma concentration–time curves are shown in
Fig. 1 and
Table 1, respectively. The results show the absorption and metabolism of p-acetaminophen in Bactrian camels are clearly changed by prior injection of lomefloxacin. Specifically, the maximum plasma concentration (C
max) of p-acetaminophen in group 2 was significantly higher than that in group 1, while the time to peak concentration (T
max) of p-acetaminophen for group 2 was much shorter than that for group 1. The results demonstrate additional differences between the 2 groups. When the CYP1A-enzyme inhibitor lomefloxacin was injected prior to the administration of p-acetaminophen, the elimination half-life (T
1/2) increased by 11.6% (
p < 0.05), area under the curve from dosing to last measurable concentration (AUC
0-t) increased by 55% (
p < 0.01), C
max increased by 27.1% (
p < 0.05), T
max decreased by 51% (
p < 0.01), total plasma clearance (CL) decreased by 26.3% (
p < 0.01) and the volume of distribution under steady-state (V
d) decreased by 10.9% (
p < 0.05). These results demonstrate that lomefloxacin has a strong inhibitory effect on the activity of the CYP1A enzyme in the Bactrian camel, decreasing the ability of the enzyme to metabolize p-acetaminophen and, thus, leading to an increase in the plasma p-acetaminophen concentration and prolonging its half-life. Thus, lomefloxacin can be used indirectly to improve the duration and intensity of p-acetaminophen action.
P-acetaminophen is a commonly used antipyretic and analgesic drug and is a specific probe substrate for the CYP1A enzyme. Pharmacokinetic studies on this drug have mainly focused on its properties in humans, porcine animals, bovine animals, and rats; however, the pharmacokinetic characteristics of p-acetaminophen in Bactrian camels have not been hitherto reported. Qiu et al. [
7] reported the following pharmacokinetic data for p-acetaminophen injected into porcine animals: T
max, 0.41 ± 0.13 h; T
1/2, 1.76 ± 0.91 h; mean residence time (MRT), 1.70 ± 0.23 h; and AUC
0-t, 23.95 ± 6.98 µg·h/mL. These data demonstrate that both the absorption and elimination of injected p-acetaminophen are faster in porcine animals than in Bactrian camels. Sawaguchi et al. [
8] studied the pharmacokinetics of p-acetaminophen in healthy cattle following oral administration and reported that T
max was 1.40 ± 0.55 h, T
1/2 was 2.41 ± 0.58 h, and the MRT was 4.70 ± 0.93 h. Yamasaki et al. [
9] reported that T
1/2 was 0.30 ± 0.02 h and the MRT was 0.40 ± 0.03 h in rats upon intramuscular injection of p-acetaminophen, revealing that it is eliminated quickly by rats. Comparing these previously reported data with those obtained in the current study (
Table 1) reveals that T
max, T
1/2, and MRT for p-acetaminophen in Bactrian camels is higher than that in pigs, cows, and rats. P-acetaminophen is absorbed and eliminated quickly by pigs, cows, and rats, whereas, it is absorbed quickly but eliminated comparatively slowly by Bactrian camels. Furthermore, the average MRT for Bactrian camels is much longer than those of the other animals. The differences observed between the biological processing of p-acetaminophen by the Bactrian camel and other animals are most likely related to its markedly different living conditions, unique biological characteristics, and the activities of its drug-metabolizing enzymes.
CYP1A enzyme activities are inhibited by many factors, including the environment, diet, and co-administration of drugs. For example, CYP1A enzyme activities are strongly inhibited by quinolones (
e.g., lomefloxacin, ciprofloxacin, and gatifloxacin), fluvoxamine, furafylline, oral contraceptives, and some traditional Chinese medicines [
1011121314]. Al-Mohizea et al. [
10] studied the effects of
Acacia catechu on the pharmacokinetics of theophylline, a CYP1A-enzyme-specific substrate, in rabbits. In their study, black catechu was given to rabbits orally for 7 consecutive days before theophylline was administered. The authors reported that rabbits pretreated with black catechu presented significant increases in theophylline C
max, T
max, and AUC
0-t, which were increased by 41.32%, 35.71%, and 15.03%, respectively, compared to those of the control group, while decreases in CL, V
d, and T
1/2 were observed. Their results indicated that pretreatment with black catechu decreases the metabolic activity of CYP1A, leading to an increase in bioavailability and a decrease in the oral clearance of theophylline due to the inhibition of CYP1A. Furthermore, Regmi et al. [
13] investigated the CYP1A-inhibiting effects of the fluoroquinolones ofloxacin, orbifloxacin, ciprofloxacin, enrofloxacin, and norfloxacin by assessing hepatic microsomes from Beagles. They reported that all of the fluoroquinolones studied inhibited CYP1A in a non-competitive manner and that several altered CYP1A activity
via mechanism-based inhibition that is irreversible and cumulative. Thus, prolonged treatment with these substances may inhibit CYP1A activity in a manner that can result in serious drug-drug interactions [
12]. Wijnands et al. [
14] reported that total body clearance of theophylline in humans was significantly decreased by enoxacin, ciprofloxacin, and pefloxacin. Furthermore, Intorre et al. [
11] studied the influence of enrofloxacin on theophylline steady-state pharmacokinetics in Beagles and observed that theophylline clearance and its concentration-time profile were significantly changed upon co-administration of enrofloxacin.
In the current study, we used p-acetaminophen and lomefloxacin as a substrate and an inhibitor of CYP1A-enzyme, respectively, to investigate the activity of the CYP1A enzyme in the Bactrian camel and its effect on the pharmacokinetic characteristics of p-acetaminophen. Most of the pharmacokinetic parameters of the p-acetaminophen in Bactrian camels were significantly affected by the prior administration of lomefloxacin. The half-life of p-acetaminophen in the Bactrian camel was prolonged by 11.6%, AUC0-t was increased by 55%, Cmax was increased by 27.1%, Tmax was shortened by 51%, CL was decreased by 26.3%, and Vd was decreased by 10.9%. These data show that the generally recognized CYP1A enzyme-specific inhibitor lomefloxacin, as with other fluoroquinolones, can significantly inhibit animal CYP1A enzyme activities.
Therefore, the pharmacokinetic process associated with p-acetaminophen in the Bactrian camel is significantly different from that in other animals, and the CYP1A enzyme-specific inhibitor lomefloxacin can alter the pharmacokinetic behavior of the probe substrate in the Bactrian camel by affecting the activity of the CYP1A enzyme. The present results provide an experimental basis for more in-depth studies of CYP enzymes that are involved in the metabolism of other exogenous substances by, as well as providing a theoretical foundation for studying the biological characteristics of, the Bactrian camel and the roles of CYP enzymes in Bactrian camel metabolism.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download