INTRODUCTION
Continuous antibiotic prophylaxis (CAP) is well-known as an initial management strategy to attenuate the risk of retrograde urinary tract infection (UTI) in children with primary vesicoureteral reflux (VUR), as a significant proportion of cases resolve spontaneously over time.
12 Although CAP has been widely administered to children with primary VUR, there has been a controversy in recent decades regarding the utilization of antimicrobial prophylaxis to prevent recurrent UTI.
345678 However, several recent studies, including the Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR) trial, have demonstrated the effectiveness of CAP for primary VUR.
91011 A recently published meta-analysis on the role of antibiotic prophylaxis in children with VUR revealed that CAP is effective in preventing UTI recurrence and that the effect is more prominent in the studies with a low risk of bias.
10
Despite the benefits of CAP for VUR, the main concern regarding CAP is the increase in resistant pathogens.
91011 Long-term antibiotic prophylaxis tends to increase the risk of emergence of bacterial strains resistant to prophylactic antibiotics, especially in breakthrough UTI.
1012 Apart from the fact that CAP increases the incidence of antibiotic-resistant bacteria in children with VUR, the association between the antibiotic susceptibility on initial UTI when diagnosing VUR (index UTI) and the clinical course (including breakthrough UTI and renal scarring) is unknown. Putting it more simply, it is not well known whether UTI, renal scarring, and other consequences of VUR are more common if the index UTI is resistant to the agent used for prophylaxis. Therefore, it is vital to resolve the concern of whether bacteria resistant to the prophylactic agent in index UTI adversely affect the clinical course in children with VUR.
In this study, we assessed the impact of the susceptibility of the index UTI to prophylactic antibiotics during breakthrough UTI in children with primary VUR receiving CAP.
METHODS
We retrospectively reviewed the medical records of children with primary VUR who were diagnosed under 2 years after febrile or symptomatic UTI, with pathogens confirmed via urine culture, between January 2010 and December 2013. Among these, subjects who underwent CAP as initial management without surgical intervention being considered were included. All children were administered trimethoprim-sulfamethoxazole (TMP-SMX) as CAP regardless of the results of urine culture or resistance pattern to antibiotic agents on the index UTI. We excluded children with a history of other prophylactic agents besides TMP-SMX, ectopic ureter, megaureter, duplicated ureter, ureterocele, neurological or structural bladder abnormalities, renal failure at presentation, and a follow-up duration of less than 24 months. After applying these criteria, a total of 81 children were finally included in this study.
In our institution, the routine evaluation in children diagnosed with VUR involves renal function testing, renal ultrasonography, and a dimercaptosuccinic acid (DMSA) renal scan for urinary tract status. The follow-up regimen including the voiding cystourethrogram (VCUG) and DMSA is conducted 1 year after VUR is diagnosed. If the breakthrough UTI occurs within 1 year, follow-up studies should be performed earlier than the routine timing. A staff radiologist and a staff nuclear medicine physician respectively evaluate the VCUG and DMSA without a medical chart review.
The conventional urine culture method was used to identify the appropriate bacterial infection. Urine specimens from all patients were obtained by means of bladder catheterization. To establish a culture, 1 µL of homogenously mixed urine sample was inoculated onto a blood agar plate and a MacConkey agar plate, using a 1.45-mm diameter calibrated loop. Plates were aerobically incubated at 37°C for 18–24 hours; thereafter, colony growth was observed. All colonies on the blood agar plate were enumerated and bacterial concentration was expressed as colony-forming units (CFU) per milliliter of the patient's urine. Gram staining was performed to elucidate colony characteristics, in combination with the exact microbial identification using the VITEK 2 System (bioMérieux, Marcy L′Ètoile, France) in accordance with the manufacturer's instructions. Per this method, 100 CFU indicates the presence of 105 bacteria in 1 mL of urine; correspondingly, antimicrobial susceptibility tests (AST) were also performed using the VITEK 2 System in cases of significant growth. Inoculated AST cards were selected on the basis of Gram status and all included antibiotics were used as recommended by the Clinical Laboratory Standard Institute guidelines for each isolate.
Based on the TMP-SMX susceptibility of the index UTI, we allocated the children with VUR to a susceptible group or a resistant group. We compared patients' characteristics and clinical outcomes after CAP according to the TMP-SMX susceptibility of the index UTI. In addition, we assessed the predictive factors of breakthrough UTI in children with VUR on CAP. Clinical outcomes, including presence of breakthrough UTI, new or worsening renal scarring, and spontaneous resolution, were assessed 24 months after CAP start. The covariables included in the analysis were age at presentation, sex, VUR grade, laterality, TMP-SMX susceptibility of index UTI, initial renal scarring, and pathogen of index UTI. Breakthrough UTI was defined as fever (≥ 38°C), pyuria on urinalysis, and/or significant pathogenic bacteria. The term significant pathogenic bacteria was defined as a growth of ≥ 50,000 CFU per milliliter for catheterized or suprapubic aspirate specimens, ≥ 100,000 CFU per milliliter for clean voided specimens with a single organism. Initial renal scarring was evaluated on a DMSA scan at least 3 months after the index UTI.
Statistical analysis
Student's t-test and the χ2 test were used to compare the variables between the two groups. Univariate and multivariate Cox proportional hazards regression models were used to assess the predictive factors of breakthrough UTI in children with VUR on CAP. The statistical analysis was performed using SPSS V.18 (SPSS Inc., Chicago, IL, USA), and the statistical significance set at P < 0.05.
Ethics statement
This study was approved by the Institutional Review Board (IRB) of Kyungpook National University Hospital (IRB No. 2018-10-005). Consent to participate was waived by the IRB due to its retrospective nature and medical records were only used in this analysis.
RESULTS
Among a total of 81 children with primary VUR, the mean age at VUR diagnosis was 5.3 months and the male-to-female ratio was 67:14. Regarding age at VUR diagnosis, 64 (79.0%) were ≤ 6 months old, 11 (13.6%) were aged 6–12 months, and 6 (7.4%) were older than 12 months. The VUR grade at diagnosis was grade 1 to 5 in 1 (1.2%), 6 (7.4%), 25 (30.9%), 23 (28.4%), and 26 cases (32.1%), respectively. Bilateral VUR and initial renal scarring were observed in 42 (51.9%) and 54 (66.7%) children, respectively. The pathogens causing index UTI were
E. coli (58.0%),
Enterococcus (18.5%),
Klebsiella (8.6%),
Enterobacter (7.4%),
Proteus (5.0%), and
Pseudomonas (2.5%). Based on the susceptibility to TMP-SMX of the index UTI, 42 patients (51.9%) were classified into the susceptible group and 39 (48.1%) in the resistant group. The resistant group was noted to have more frequent high-grade reflux and non-
E. coli strains as index UTI pathogens than the susceptible group (
P = 0.044 and < 0.001, respectively). Age, sex, laterality, and initial renal scarring were not significantly different between groups (
Table 1).
Table 1
Demographics and clinical outcome according to the susceptibility of the index UTI to TMP-SMX

|
Variables |
Total patients |
Susceptible group |
Resistant group |
P value |
|
Demographics |
|
|
|
|
|
No. |
81 (100) |
42 (51.9) |
39 (48.1) |
|
|
Age, mon |
|
|
|
|
|
Median |
5.3 |
6.6 |
4.8 |
|
|
Group |
|
|
|
0.258 |
|
|
2–6 |
64 (79.0) |
30 (71.4) |
34 (87.2) |
|
|
7–11 |
11 (13.6) |
8 (19.0) |
3 (7.7) |
|
|
> 12 |
6 (7.4) |
4 (9.5) |
2 (5.1) |
|
Sex |
|
|
|
0.184 |
|
Male |
67 (82.7) |
37 (88.1) |
30 (76.9) |
|
Female |
14 (17.3) |
5 (11.9) |
9 (23.1) |
|
Highest grade of VUR |
|
|
|
0.044 |
|
Grade I |
1 (1.2) |
0 (0) |
1 (2.6) |
|
Grade II |
6 (7.4) |
6 (14.3) |
0 (0) |
|
Grade III |
25 (30.9) |
15 (35.7) |
10 (25.6) |
|
Grade IV |
23 (28.4) |
10 (23.8) |
13 (33.3) |
|
Grade V |
26 (32.1) |
11 (26.2) |
15 (38.5) |
|
Bilateral VUR |
42 (51.9) |
21 (50.0) |
21 (53.8) |
0.729 |
|
Initial renal scarring |
54 (66.7) |
29 (69.0) |
25 (64.1) |
0.637 |
|
Index UTI pathogen |
|
|
|
< 0.001 |
|
Escherichia coli
|
47 (58.0) |
34 (81.0) |
13 (33.3) |
|
Enterococcus
|
15 (18.5) |
1 (2.4) |
14 (35.9) |
|
Klebsiella
|
7 (8.6) |
1 (2.4) |
6 (15.4) |
|
Enterobacter
|
6 (7.4) |
3 (7.1) |
3 (7.7) |
|
Proteus
|
4 (5.0) |
3 (7.1) |
1 (2.6) |
|
Pseudomonas
|
2 (2.5) |
0 (0) |
2 (5.1) |
|
Clinical outcome |
|
|
|
|
|
Breakthrough UTI |
34 (42.0) |
13 (31.0) |
21 (53.8) |
0.037 |
|
Renal scarring f/u DMSA scan |
58 (71.6) |
29 (69.0) |
29 (74.4) |
0.596 |
|
New or worsening renal scarring |
6 (7.4) |
0 (0) |
6 (15.4) |
0.053 |
|
Spontaneous resolution |
26 (32.1) |
18 (42.9) |
8 (20.5) |
0.031 |

During the 24-month follow-up period, 34 children (42.0%) had at least 1 breakthrough febrile UTI. Among these, the breakthrough UTI was observed in 31.0% (13/42) of children in the susceptible group and 53.8% (21/39) of children in the resistant group (P = 0.037). New/worsening of renal scarring was observed in 0% in the susceptible group and 15.4% (6/39) in the resistance group at the 24-month follow-up, although the difference was not found to be statistically significant (P = 0.053). In addition, spontaneous resolution of VUR was observed in 42.9% (18/42) children in the susceptible group and in 20.5% (8/39) children in the resistant group during the two years after CAP (P = 0.031).
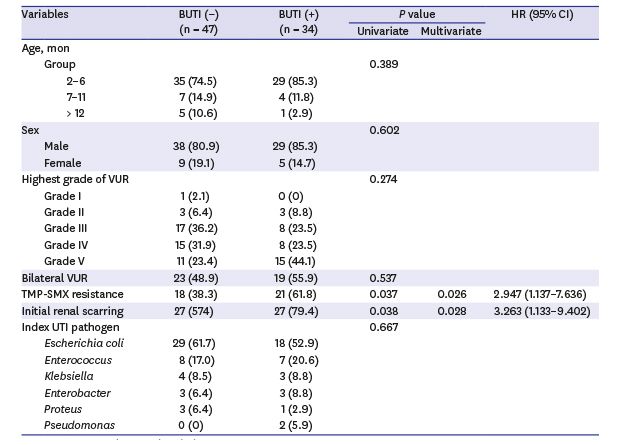
Table 2 shows the results of univariate and multivariate Cox proportional hazards analyses of the predictive factors associated with breakthrough UTI after CAP in children with VUR. On univariate analysis, TMP-SMX resistance and initial renal scarring were significantly associated with breakthrough UTI (
P = 0.037 and
P = 0.038, respectively). TMP-SMX resistance of the index UTI pathogen and presence of initial renal scarring still remained the significant risk factors for breakthrough UTI on multivariate analysis (hazard ratio [HR], 2.947; 95% confidence interval [CI], 1.137–7.636;
P = 0.026 and HR, 3.263; 95% CI, 1.133–9.402;
P = 0.028, respectively). Other factors, including age, sex, VUR grade, laterality, and index UTI pathogen, were not associated with breakthrough UTI.
Table 2
Predictive factors associated with breakthrough urinary tract infection

|
Variables |
BUTI (−) (n = 47) |
BUTI (+) (n = 34) |
P value |
HR (95% CI) |
|
Univariate |
Multivariate |
|
Age, mon |
|
|
|
|
|
|
Group |
|
|
0.389 |
|
|
|
|
2–6 |
35 (74.5) |
29 (85.3) |
|
|
7–11 |
7 (14.9) |
4 (11.8) |
|
|
> 12 |
5 (10.6) |
1 (2.9) |
|
Sex |
|
|
0.602 |
|
|
|
Male |
38 (80.9) |
29 (85.3) |
|
Female |
9 (19.1) |
5 (14.7) |
|
Highest grade of VUR |
|
|
0.274 |
|
|
|
Grade I |
1 (2.1) |
0 (0) |
|
Grade II |
3 (6.4) |
3 (8.8) |
|
Grade III |
17 (36.2) |
8 (23.5) |
|
Grade IV |
15 (31.9) |
8 (23.5) |
|
Grade V |
11 (23.4) |
15 (44.1) |
|
Bilateral VUR |
23 (48.9) |
19 (55.9) |
0.537 |
|
|
|
TMP-SMX resistance |
18 (38.3) |
21 (61.8) |
0.037 |
0.026 |
2.947 (1.137–7.636) |
|
Initial renal scarring |
27 (574) |
27 (79.4) |
0.038 |
0.028 |
3.263 (1.133–9.402) |
|
Index UTI pathogen |
|
|
0.667 |
|
|
|
Escherichia coli
|
29 (61.7) |
18 (52.9) |
|
Enterococcus
|
8 (17.0) |
7 (20.6) |
|
Klebsiella
|
4 (8.5) |
3 (8.8) |
|
Enterobacter
|
3 (6.4) |
3 (8.8) |
|
Proteus
|
3 (6.4) |
1 (2.9) |
|
Pseudomonas
|
0 (0) |
2 (5.9) |

DISCUSSION
Several patient factors including initial grade, age at presentation, mode of clinical presentation, sex, laterality, and ureteral anatomy are known to be related to the clinical outcomes such as spontaneous resolution and breakthrough UTI.
13 However, a few studies have reported on the effect of pathogen characteristics, such as susceptibility to prophylactic agents at index UTI, on clinical outcomes in children with VUR.
1415 In this era of proven effectiveness of CAP in children with primary VUR,
10 the impact of the susceptibility of the index UTI pathogen to a prophylactic agent on the clinical outcome of CAP needs to be clarified. In this study, we retrospectively assessed this point and revealed that the TMP-SMX resistant group on the index UTI showed poorer clinical outcomes, including breakthrough UTI and spontaneous resolution, than did the susceptible group. In addition, the susceptibility of the index UTI was an independent predictive factor of breakthrough UTI on multivariate analysis.
A recently published meta-analysis that included eight studies showed the effectiveness of CAP for preventing recurrence of UTI, although six of the studies excluding the Prevention of Recurrent Urinary Tract Infection in Children with Vesicoureteric Reflux and Normal Renal Tracts (PRIVENT)
9 and RIVUR
11 trials were determined as having a high risk of bias.
10 A meta-analysis revealed that CAP significantly reduced the risk of febrile/symptomatic urinary tract infections compared to no treatment or placebo in children with VUR.
10 However, the chief concern regarding the use of CAP in children with VUR is an increased risk of infection caused by antibiotic-resistant bacteria. Two studies with a low risk of bias, both of which included the PRIVENT and RIVUR trials, found a higher incidence of UTI caused by resistant pathogens in children receiving CAP than in those receiving placebo (PRIVENT, 67 vs. 25%; RIVUR, 68 vs. 25%, respectively).
911 Other similar studies have also reported that a major concern in the prophylaxis group was recurrent infection caused by bacteria that were resistant to the antibiotic used for prophylaxis.
357 A meta-analysis revealed that CAP was associated with an increased risk of antibiotic-resistant bacteria (pooled odds ratio [OR], 8.75; 95% CI, 3.52–21.73;
P < 0.001).
10
Although several studies have found a significant reduction in repeat UTI in children receiving CAP compared to those receiving placebo, antimicrobial prophylaxis inevitably increases the risk that a later infecting bacterial strain will be resistant to antibiotics. However, previous trials have argued whether CAP has any benefits when a child's index UTI is caused by bacteria resistant to prophylactic antibiotics.
91115 A recent analysis that used data from the RIVUR trial proposed that there was no significant difference in UTI recurrence between those with TMP-SMX–resistant index UTI versus TMP-SMX–susceptible UTI in children who were treated with CAP using TMP-SMX.
15 They showed that the TMP-SMX resistance status of the index febrile/symptomatic UTI was not associated with recurrence of febrile/symptomatic UTI among those treated with CAP. Among the prophylactic group, 7 (13%) of 55 with of TMP-SMX–resistant index UTI had recurrent UTI, whereas 27 (12%) of 223 with TMP-SMX–susceptible index UTI had recurrent UTI. In other words, they suggested that the benefit of CAP occurred irrespective of whether the index UTI was sensitive to prophylactic antibiotics. Although they had advantage of the robust prospective data collection seen in the RIVUR trial,
15 it also shows the potential pitfalls of underpowered, nonrandomized subgroup analysis.
16 In addition, the RIVUR trial has several limitations. More than 90% of children with VUR in the RIVUR trial had reflux of grade III or less. In addition, less than 5% of children with renal scarring at baseline as documented using the DMSA scan were included in the study.
11 Although scarring rates vary widely in patients, evidence of renal scarring is present in 10%–40% of children with symptomatic VUR; this scarring is due to congenital dysplasia and/or acquired post-infectious damage.
17 Well-designed studies regarding the impact of antibiotic susceptibility of the index UTI pathogens on clinical outcomes in children with VUR are lacking; therefore, the current clinical practice has been widely questioned.
Although the PRIVENT trial did not report the impact of index UTI resistance on recurrence for the CAP group specifically, they reported that CAP has a higher preventive effect in children with TMP-SMX sensitive index UTI than in those with TMP-SMX resistant index UTI.
9 The data obtained in our study are consistent with the data obtained in the PRIVENT trial. Especially, our results indicate that CAP in children with VUR with index UTI caused by the strains resistant to prophylactic agents shows poor outcomes, including a breakthrough UTI rate of > 50% and a low rate of spontaneous resolution (20%) over a two-year study period. This result indicates that the choice of management plan in children with VUR should be made carefully, considering the susceptibility of index UTI pathogen; this will prevent breakthrough UTI and subsequent worsening of renal scarring. Similar to this study, Park et al.
14 also reported an emphasis on the importance of bacterial pathogenic factors. Although they did not report on the susceptibility state and exact explanation for mechanisms underlying this finding, they found that initial infection by a non-
E. coli strain significantly increased the risk of breakthrough UTI.
In this study, we also found that initial renal scarring is a significant predictor of breakthrough UTI in children with VUR receiving CAP. These findings are similar to those of previous studies.
181920 Renal scarring has been accepted as a predictive factor for breakthrough infection in children with primary VUR.
181920 Nakamura et al.
18 and Shiraishi et al.
19 showed that an abnormal renal scan as detected using DMSA is a prominent risk factor for breakthrough infection in patients with primary VUR who were treated with antibiotic prophylaxis. They suggested that CAP as standard treatment may be insufficient in a specific subgroup of children with an abnormal DMSA scan. In our study, we confirmed initial renal scarring as a risk factor for breakthrough infection using multivariate analysis (HR, 3.263; 95% CI, 1.133–9.402;
P = 0.028). In addition, a previous meta-analysis of the DMSA literature revealed that the incidence of renal scarring following acute pyelonephritis varied according to region, from 16.7% (Middle East) to 58.4% (Asia) in terms of per renal units.
21 This fact supports the cause of a high incidence of renal scarring as seen on DMSA in this study. Furthermore, it strengthens the need for a difference in decisions regarding treatment policies according to region and/or race in children with VUR for more precise treatment.
Till date, CAP has been deemed generally prudent in children with VUR. This study demonstrates a significant difference in breakthrough UTI regarding the index UTI susceptibility to prophylactic agent (TMP-SMX) among children with VUR receiving CAP. The susceptibility of the index UTI to prophylactic antibiotics and initial renal scarring were significant predictors of breakthrough UTI. This result supported that the children with an index UTI that was resistant to a prophylactic antibiotic (TMP-SMX) might show relatively fewer benefits from CAP. In addition, the value of antibiotic susceptibility in terms of pathogenic factor has not yet been well identified. Physicians should consider the susceptibility of the index UTI pathogen to prophylactic antibiotics to determine the most effective and beneficial management plan in children with primary VUR.
This study had several limitations. This was a retrospective study based on a review of records of children who were treated at a single institution. Although the grade was not identified as a predictive factor of breakthrough UTI in multivariable analysis, high proportion of high grade VUR may be a part of limitations in this retrospective study. The relatively small numbers in this study may have limited the ability to detect an increased risk of breakthrough UTI in those with high grade VUR. A selection bias regarding the initial choice of management plan was unavoidable, despite the children included in this study underwent medical management including CAP during the study period. In addition, fecal culture, which is the main investigation that provides data on the mechanism of ascending infection, was not performed in all children in this retrospective study. These limitations highlight the need for more studies with standardized designs and outcome-reporting methods in the future.
In conclusion, the susceptibility of initial UTI to prophylactic antibiotics is a risk factor of breakthrough UTI and is associated with poor clinical outcome in children with primary VUR receiving CAP. In this era of precision medicine, the susceptibility of initial UTI to prophylactic antibiotics may help physicians and families arrive at more informed decisions about the choice of management plan in children with primary VUR.