Abstract
Inflammatory bowel disease (IBD) is a chronic inflammatory disease of the gastrointestinal tract with an unknown etiology and pathogenesis. The incidence and prevalence of IBD are increasing rapidly in Korea. Approximately one-third of patients with IBD appear to develop extra-intestinal manifestations with the skin being one of the most commonly affected organs. They may precede, occur simultaneously, or follow the diagnosis of IBD. In addition, they may parallel with the luminal symptoms or independent from the disease activity of IBD. This review outlines the skin manifestations associated with IBD and discusses their management. Skin manifestations should be managed in close collaboration with a dermatologist.
Figures and Tables
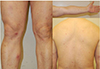
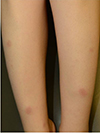 | Fig. 1Erythema nodosum. Tender, bilateral, erythematous nodules and plaques on the anterior aspect of the lower extremities. |
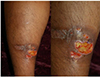 | Fig. 2Pyoderma gangrenosum. Ulcer shows an edematous pale border with granulation tissue on the leg. |
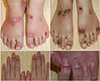 | Fig. 4Acrodermatitis enteropathica. (A) Multiple flaccid bullae and vesicles on dorsum of the feet. (B) Hyperpigmented macules and patches with scale resolved on the dorsum of the feet. (C, D) Paronychia. |
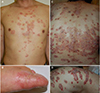 | Fig. 5TNF-α inhibitor treatment-induced skin manifestations. (A, B) Small plaque psoriasiform lesions scattered over the trunk and palmoplantar pustular psoriasiform lesions following TNF-α inhibitor therapy. (C, D) Generalized blistering skin lesions on the trunk of a 40-year-old woman treated for ulcerative colitis with infliximab. TNF-α, tumor necrosis factor-alpha. |
References
2. Lee JW, Im JP, Cheon JH, Kim YS, Kim JS, Han DS. Inflammatory bowel disease cohort studies in Korea: present and future. Intest Res. 2015; 13:213–218.

3. Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005; 129:827–836.

4. Veloso FT, Carvalho J, Magro F. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J Clin Gastroenterol. 1996; 23:29–34.
5. Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011; 106:110–119.

6. Guest GD, Fink RL. Metastatic Crohn's disease: case report of an unusual variant and review of the literature. Dis Colon Rectum. 2000; 43:1764–1766.
7. Marzano AV, Borghi A, Stadnicki A, Crosti C, Cugno M. Cutaneous manifestations in patients with inflammatory bowel diseases: pathophysiology, clinical features, and therapy. Inflamm Bowel Dis. 2014; 20:213–227.
8. Sandborn WJ. Evidence-based treatment algorithm for mild to moderate Crohn's disease. Am J Gastroenterol. 2003; 98:12 Suppl. S1–S5.

9. Ploysangam T, Heubi JE, Eisen D, Balistreri WF, Lucky AW. Cutaneous Crohn's disease in children. J Am Acad Dermatol. 1997; 36(5 Pt 1):697–704.

10. Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn's disease: a review. J Eur Acad Dermatol Venereol. 2008; 22:1033–1043.

11. Lee KG, Koh BT, Kwon ES, Myung KB, Cheong SH. Crohn's disease initially presenting as vulvar swelling. Korean J Dermatol. 2018; 56:49–52.
12. Bender-Heine A, Grantham JT, Zaslau S, Jansen R. Metastatic Crohn disease: a review of dermatologic manifestations and treatment. Cutis. 2017; 99:E33–E40.
13. Huang W, McNeely MC. Neutrophilic tissue reactions. Adv Dermatol. 1997; 13:33–64.
14. Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2015; 21:1982–1992.

15. Farhi D, Cosnes J, Zizi N, et al. Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2402 patients. Medicine (Baltimore). 2008; 87:281–293.
16. Yang BR, Choi NK, Kim MS, et al. Prevalence of extraintestinal manifestations in Korean inflammatory bowel disease patients. PLoS One. 2018; 13:e0200363.

17. Vavricka SR, Rogler G, Gantenbein C, et al. Chronological order of appearance of extraintestinal manifestations relative to the time of IBD diagnosis in the Swiss inflammatory bowel disease cohort. Inflamm Bowel Dis. 2015; 21:1794–1800.

18. Yüksel I, Başar O, Ataseven H, et al. Mucocutaneous manifestations in inflammatory bowel disease. Inflamm Bowel Dis. 2009; 15:546–550.

19. Timani S, Mutasim DF. Skin manifestations of inflammatory bowel disease. Clin Dermatol. 2008; 26:265–273.

20. Clayton TH, Walker BP, Stables GI. Treatment of chronic erythema nodosum with infliximab. Clin Exp Dermatol. 2006; 31:823–824.

21. Ortego-Centeno N, Callejas-Rubio JL, Sanchez-Cano D, Caballero-Morales T. Refractory chronic erythema nodosum successfully treated with adalimumab. J Eur Acad Dermatol Venereol. 2007; 21:408–410.

22. Trost LB, McDonnell JK. Important cutaneous manifestations of inflammatory bowel disease. Postgrad Med J. 2005; 81:580–585.

23. Greuter T, Bertoldo F, Rechner R, et al. Extraintestinal manifestations of pediatric inflammatory bowel disease: prevalence, presentation, and anti-TNF treatment. J Pediatr Gastroenterol Nutr. 2017; 65:200–206.

24. Rothfuss KS, Stange EF, Herrlinger KR. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J Gastroenterol. 2006; 12:4819–4831.

25. Crowson AN, Mihm MC Jr, Magro C. Pyoderma gangrenosum: a review. J Cutan Pathol. 2003; 30:97–107.
26. Feliciani C, De Simone C, Amerio P. Dermatological signs during inflammatory bowel diseases. Eur Rev Med Pharmacol Sci. 2009; 13:Suppl 1. 15–21.
27. Menachem Y, Gotsman I. Clinical manifestations of pyoderma gangrenosum associated with inflammatory bowel disease. Isr Med Assoc J. 2004; 6:88–90.
28. Agarwal A, Andrews JM. Systematic review: IBD-associated pyoderma gangrenosum in the biologic era, the response to therapy. Aliment Pharmacol Ther. 2013; 38:563–572.

29. Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006; 55:505–509.

30. Lee JH, Chang IK, Lee HE, et al. Treatment of recalcitrant pyoderma gangrenosum with ulcerative colitis by adalimumab injection. Ann Dermatol. 2017; 29:260–262.

31. Ali M, Duerksen DR. Ulcerative colitis and Sweet's syndrome: a case report and review of the literature. Can J Gastroenterol. 2008; 22:296–298.

32. Vavricka SR, Manser CN, Hediger S, et al. Periodontitis and gingivitis in inflammatory bowel disease: a case-control study. Inflamm Bowel Dis. 2013; 19:2768–2777.
33. Calobrisi SD, Mutasim DF, McDonald JS. Pyostomatitis vegetans associated with ulcerative colitis. Temporary clearance with fluocinonide gel and complete remission after colectomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995; 79:452–454.
34. Kim TH, Kim SC. Pyodermatitis-pyostomatitis vegetans associated with Crohn's disease. Ann Dermatol. 2015; 27:624–625.

35. Bens G, Laharie D, Beylot-Barry M, et al. Successful treatment with infliximab and methotrexate of pyostomatitis vegetans associated with Crohn's disease. Br J Dermatol. 2003; 149:181–184.

36. Campisi G, Compilato D, Cirillo N, et al. Oral ulcers: three questions on their physiopathology. Minerva Stomatol. 2007; 56:293–302.
37. Löfberg R, Louis EV, Reinisch W, et al. Adalimumab produces clinical remission and reduces extraintestinal manifestations in Crohn's disease: results from CARE. Inflamm Bowel Dis. 2012; 18:1–9.

38. Vavricka SR, Gubler M, Gantenbein C, et al. Anti-TNF treatment for extraintestinal manifestations of inflammatory bowel disease in the Swiss IBD cohort study. Inflamm Bowel Dis. 2017; 23:1174–1181.

39. Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018; 154:1417–1423.
40. Kim M, Choi KH, Hwang SW, Lee YB, Park HJ, Bae JM. Inflammatory bowel disease is associated with an increased risk of inflammatory skin diseases: a population-based cross-sectional study. J Am Acad Dermatol. 2017; 76:40–48.

41. Asarch A, Barak O, Loo DS, Gottlieb AB. Th17 cells: a new paradigm for cutaneous inflammation. J Dermatolog Treat. 2008; 19:259–266.

42. Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology. 2011; 140:1704–1712.

43. Whitlock SM, Enos CW, Armstrong AW, et al. Management of psoriasis in patients with inflammatory bowel disease: from the medical board of the national psoriasis foundation. J Am Acad Dermatol. 2018; 78:383–394.

44. Wittig BM. Drug evaluation: CNTO-1275, a mAb against IL-12/IL-23p40 for the potential treatment of inflammatory diseases. Curr Opin Investig Drugs. 2007; 8:947–954.
45. Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013; 168:844–854.
46. Menter A, Papp KA, Gooderham M, et al. Drug survival of biologic therapy in a large, disease-based registry of patients with psoriasis: results from the psoriasis longitudinal assessment and registry (PSOLAR). J Eur Acad Dermatol Venereol. 2016; 30:1148–1158.

47. Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the psoriasis longitudinal assessment and registry (PSOLAR). J Drugs Dermatol. 2015; 14:706–714.
48. Danese S, Semeraro S, Papa A, et al. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol. 2005; 11:7227–7236.

49. Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007; 56:116–124.

50. Lim YS, Lee MW, Choi JH, Sung KJ. Original articles : the clinical study of zinc deficiency presented as a skin manifestation of acrodermatitis enteropathica. Korean J Dermatol. 2000; 38:155–162.
51. Rahier JF, Buche S, Peyrin-Biroulet L, et al. Severe skin lesions cause patients with inflammatory bowel disease to discontinue anti-tumor necrosis factor therapy. Clin Gastroenterol Hepatol. 2010; 8:1048–1055.

52. Cullen G, Kroshinsky D, Cheifetz AS, Korzenik JR. Psoriasis associated with anti-tumour necrosis factor therapy in inflammatory bowel disease: a new series and a review of 120 cases from the literature. Aliment Pharmacol Ther. 2011; 34:1318–1327.

53. Moon IJ, Moon HR, Noh TK, et al. Multiple cases of psoriasiform skin lesions followinganti-TNF-α therapy: a single center experience. J Korean Soc Psori. 2018; 13:14–18.
54. Sfikakis PP, Iliopoulos A, Elezoglou A, Kittas C, Stratigos A. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005; 52:2513–2518.

55. Harbord M, Annese V, Vavricka SR, et al. The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis. 2016; 10:239–254.

56. Fiorino G, Danese S, Pariente B, Allez M. Paradoxical immune-mediated inflammation in inflammatory bowel disease patients receiving anti-TNF-α agents. Autoimmun Rev. 2014; 13:15–19.

57. Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012; 143:390–399.

58. Askling J, Fahrbach K, Nordstrom B, Ross S, Schmid CH, Symmons D. Cancer risk with tumor necrosis factor alpha (TNF) inhibitors: meta-analysis of randomized controlled trials of adalimumab, etanercept, and infliximab using patient level data. Pharmacoepidemiol Drug Saf. 2011; 20:119–130.

59. McKenna MR, Stobaugh DJ, Deepak P. Melanoma and non-melanoma skin cancer in inflammatory bowel disease patients following tumor necrosis factor-α inhibitor monotherapy and in combination with thiopurines: analysis of the Food and Drug Administration adverse event reporting system. J Gastrointestin Liver Dis. 2014; 23:267–271.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download