INTRODUCTION
Ovarian cancer is the leading cause of death among patients with gynaecological malignancies [
12]. Typically, there is an initial encouraging response to platinum- and taxane-based chemotherapy and surgery, but around 70% of those with advanced disease will relapse, and the number of patients living in a palliative situation increase [
34]. Paclitaxel, pegylated liposomal doxorubicin and topotecan are currently approved to treat the subset of patients with platinum resistant ovarian cancer, but the response rate is poor and the toxicity high. The overall survival (OS) for these patients is usually around 12 months [
5].
Programmed cell death-ligand 1 (PD-L1) expression is associated with poor prognosis, and it is known that PD-L1 promotes progression of ovarian cancer [
67]. A phase II clinical trial demonstrated that nivolumab, a programmed cell death protein 1 (PD-1) receptor blocker, was well-tolerated and offered a disease control rate of 45% [
8]. A recent update of this patient cohort showed a continued clinical benefit, even after drug discontinuation [
9]. Pembrolizumab, also a PD-1 blocker that resembles nivolumab, is currently being tested on patients with ovarian cancer. Early results show good tolerance and promising disease control [
1]. There are now around 100 clinical studies testing PD-1 blockers, and several of them are focusing on ovarian cancer [
10].
Mild adverse events are known to be associated with immunotherapy. Most frequent events are fatigue, rash, pruritus, diarrhoea, and nausea. However, more serious events such as pneumonitis and/or interstitial pulmonary disease, haemorrhagic colitis, and endocrine disorders have also been observed [
8]. Still, compared to standard chemotherapy, the rate of serious adverse events is much less frequent when using PD-1 inhibitors [
11]. In Norway, off-label PD-1 inhibitors are only offered to patients with platinum resistant ovarian cancer in private hospitals. In this study, we evaluated the toxicity and clinical efficacy of a PD-1 inhibitor on patients with platinum resistant ovarian cancer.
MATERIALS AND METHODS
This quality control study included all patients with platinum-resistant ovarian cancer patients treated with a PD-1 inhibitor at Aleris Cancer Centre between November 2015 and February 2017. Platinum-resistant ovarian cancer was defined as recurrence of disease <6 months after completion of platinum-based therapy. In the platinum resistant phase, all patients received chemotherapy according to Norwegian guidelines including paclitaxel with- or without bevacizumab, pegylated liposomal doxorubicin, topotecan, and gemcitabine. All patients had measurable disease. The study was approved by the local Primary Protection Council (2017/8669). Live patients had signed a letter of consent, while a waiver was issued for all those deceased.
Baseline screening prior to treatment included clinical examination, Eastern Cooperative Oncology Group (ECOG) status, a full blood count, liver enzymes, renal function, thyroid-stimulating hormone/T3/T4, cancer antigen 125 (CA-125), C-reactive protein, and Albumin. All had a baseline contrast-enhanced computed tomography (CT) examination of the thorax, abdomen, and pelvis within 4 weeks prior to treatment. In 7 patients, concomitant bevacizumab was administered by the local hospital, 10 mg/kg every second week, or 15 mg/kg every third week.
1. Treatment protocol
Patients received intravenous nivolumab 3 mg per kg bodyweight every second week, 6 times before response evaluation. Infusion time was 60 minutes. Evaluation of clinical status, blood test results, and adverse effects was performed prior to every treatment session. Treatment cycles were scheduled until disease progression or occurrence of intolerable adverse events.
2. Response evaluation
CT and clinical response evaluation was scheduled every 12th week until disease progression. Tumor response assessment was performed according to response evaluation criteria in solid tumors (RECIST) v1.1 and immune response RECIST (irRECIST) criteria. Immune-related progressive disease (irPD) was defined as 20% (minimum 5 mm) increase in total measured tumor burden compared with nadir or progression of non-target lesions or new lesions. Confirmation of progression was required minimum 4 weeks after the first irPD assessment. RECIST 1.1 defines progression as appearance of new lesions and/or 20% increase in tumor burden.
The radiological response evaluation at the time of treatment was not systematical according to RECIST criteria, and when irRECIST were introduced, subsequent CTs were evaluated accordingly [
12]. One dedicated radiologist re-evaluated external CTs, and assessed whether there had been a radiological progression or not. We retrospectively evaluated all CTs for complete subclassification according to irRECIST and RECIST 1.1.
Disease control rate was the proportion of patients with complete response, partial response, or stable disease [
8]. The objective response rate is defined as the proportion of patients with complete response or partial response.
3. Adverse events
Prior to each treatment, peripheral blood was sampled, and the oncologist interviewed patients every second week for possible adverse events. If relevant, patients reported adverse events if they were occurring in between 2 treatments. The severity was evaluated based on blood sampling and clinical symptoms, and was reported according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.0; mild (grade 1), moderate (grade 2), severe (grade 3), life-threatening (grade 4), or lethal (grade 5).
4. Outcomes
The disease control rate and the rate of any adverse events were the primary endpoints. Secondary endpoints were progression free survival (PFS) and OS.
5. Statistical methods
The follow-up time was the number of weeks between treatment-start and the 13th of March 2018, at which point we verified the vital status for all patients using the national electronic death registry as the reference. Survival was estimated according to the Kaplan-Meier method. For OS, weeks-to-event was the number of weeks from treatment-start until death from any cause. For PFS, weeks-to-event was the number of weeks between treatment-start and the date of the last radiological response evaluation according to irRECIST, or the date of clinical progression or death, whichever occurred first. Patients lost to follow-up prior to documented disease progression are censored at the last tumor assessment date when the patient is known to be progression-free. The duration of platinum resistant cancer was the number of months from the onset of resistance until the start of PD-1 inhibitor treatment. The number of treatment lines included all treatment lines, as well as adjuvant treatment.
For descriptive data, we used both mean and median values with bias-corrected accelerated bootstrapped 95% confidence intervals (CIs) or interquartile ranges (IQRs). Hazard ratios (HRs) with 95% CI was calculated to evaluate the impact of prognostic factors upon survival. Odds ratio (OR) was calculated to consider the impact of bevacizumab on the rate of side-effects. Two-sided p
-values were given, and not corrected for multiple testing. IBM SPSS Statistics v 23 (IBM Corp., Armonk NY, USA) and MedCalc statistical software v.14.8.1 (MedCalc Software, Ostend, Belgium) were used for statistical analysis in
Figs. 1 and
2. GraphPad Prism v.6.0 (GraphPad Software, La Jolla, CA, USA) was used to make
Fig. 3.
 | Fig. 1Overall survival.
|
 | Fig. 2Progression-free survival.
|
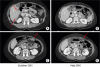 | Fig. 3
A 56-year-old patient with no clinical symptoms and ECOG 0. CT, baseline (A) and after (B) response evaluation demonstrated response to therapy as the liver metastases disappeared, and the peritoneal metastases shrunk (red arrows). Due to alterations in the total tumor burden, the patient was classified as stable disease according to irRECIST.
CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; irRECIST, immune response RECIST.

|
RESULTS
Twenty patients were eligible for treatment during the study period of which 18 started with PD-1 treatment. The other 2 declined treatment. Fourteen patients underwent at least one radiological response evaluation while 4 patients died before the first response evaluation. The latter 18 were included for further analysis. Seventeen had ovarian cancer, and 1 had fallopian tube cancer. The median number of previous treatment lines with standard chemotherapy was 3.5 (mean, 3.7; range, 2–6) and 50% had ≥4 previous treatment lines. The median number of treatment lines in the platinum-resistant phase was 2.5 (range, 1–3). The median duration of platinum-resistant disease prior to PD-1 treatment was 13 months (IQR, 7–20; range, 1–40). Four patients were ECOG 0 (22%), 12 were ECOG 1 (67%), and 2 were ECOG 2 (11%). Patient characteristics are described in
Table 1. The median follow-up time was 30 weeks (range, 3–123). Histological subgroups were: high grade serous/papillary 14 (78%), carcinosarcoma 1 (6%), mucinous/endometroid 1 (6%), clear cell 1 (6%), and low differentiated 1 (6%).
Table 1
Patient characteristics

|
Characteristics |
Median |
95% CI |
Mean |
95% CI |
Range |
|
Age (yr) |
61 |
57–66 |
61 |
58–65 |
45–78 |
|
Weight (kg) |
65 |
59–73 |
67 |
62–73 |
51–97 |
|
Hemoglobin (g/dL) |
12.2 |
11–13 |
11.7 |
11–13 |
7–14 |
|
Albumin (g/dL) |
39 |
37–41 |
38 |
36–40 |
25–43 |
|
CA-125 (IU/mL) |
282 |
106–425 |
723 |
342–1,306 |
25–4,785 |
|
LDH (IU/L) |
251 |
209–274 |
256 |
224–293 |
166–431 |
|
CRP (mg/L) |
8 |
3–31 |
29 |
10–57 |
1–263 |

The median number of PD-1 inhibitor treatments was 6 (IQR, 3–11; range, 1–60), and the median duration of treatment was 12 weeks (IQR, 6–22; range, 2–128). The median duration of treatment for patients receiving bevacizumab or not, was 16 weeks (IQR, 12–30) and 8 weeks (IQR, 4–18), respectively (p=0.332) After 12 weeks, the ECOG status was improved in 1 patient, unchanged in 9, and worse in 8.
Disease control rate according to irRECIST was 44% (8 out of 18, 95% CI=19–87) as 8 showed stable disease, 6 showed progressive disease and 4 died before the first radiological response evaluation (
Fig. 3). The objective response rate was 0% as none demonstrated partial- or complete response. Two patients with radiologically stable disease worsened from a clinical point of view. The remaining 6 with radiological stable disease had stable or improved ECOG status. Four out of seven (57%) patients receiving concomitant bevacizumab showed stable disease. The disease control rate according to RECIST 1.1 was 39% (7 out of 18, 95% CI=16–80) as 7 patients showed stable disease and 7 patients showed progressive disease. None showed complete- or partial response.
1. OS and PFS
The median OS was 30 weeks (95% CI=15–45; range, 3–123) and the median PFS was 15 weeks (95% CI=13–17). The median OS for patients receiving bevacizumab or not, was 71 weeks (95% CI=27–115) and 23 weeks (95% CI=10–36), respectively (p=0.319). PFS for patients receiving bevacizumab or not, was 21 weeks (95% CI=3–39) and 15 weeks (7–23), respectively (p=0.442).
2. Prognostic factors
Higher serum albumin was associated with improved survival, as 1 unit (1 g/dL) of higher serum albumins resulted in 24% better OS (HR=0.76; 95% CI=0.63–0.92; p=0.004). Also, 1 extra month of platinum resistant disease resulted in 8% worsened OS (HR=1.08; 95% CI=1.03–1.14; p=0.004).
3. Adverse events
The rate of grade 2–5 adverse events was 28% (5 out of 18) as seen in
Table 2. Four events (22%) were caused by PD-1 treatment, of which treatment was discontinued for 2 patients with grade 3 (
Fig. 4). Further, 2 cases with grade 2 events continued PD-1 treatment after administration of steroids. There was a tendency towards increased toxicity when using concomitant bevacizumab (OR=3.4; 95% CI=0.4–28.0; p=0.266). Two out of the seven (35%) patients receiving concomitant bevacizumab experienced grade 3 adverse events while 1 patient died (grade 5) from intestinal perforation.
Table 2
Adverse events in patients receiving nivolumab with- and without bevacizumab

|
Adverse events |
Nivolumab |
Comment |
Total |
|
With bevacizumab |
Without bevacizumab |
|
No |
5 |
9 |
|
14 |
|
Grade |
|
|
|
|
|
1 |
NA |
NA |
Not systematically reported |
NA |
|
2 |
1 |
1 |
Pancytopenia (1) and skin rash (1) |
2 |
|
3 |
1 |
1 |
Hepatitis (1) and pneumonitis (1) |
2 |
|
4 |
0 |
0 |
NA |
0 |
|
5 (death) |
1 |
0 |
Intestinal perforation causing peritonitis, sepsis and death |
1 |

 | Fig. 4
During the first response-evaluation, CT revealed stable disease, but several unspecific opacities (*) in the right lung (A). The condition was interpreted as pneumonitis and treated with prednisolon. Eight weeks later a CT demonstrated a complete regress of the pneumonitis (B). Later on, the patient developed once again pneumonitis, and treatment was discontinued.
CT, computed tomography.

|
DISCUSSION
In this study, the disease control rate was 44% when using irRECIST and 39% when using RECIST 1.1. Two patients had to discontinue treatment due to toxicity. The reason for the slight discrepancy in disease control rates using the 2 systems is due to differences in the definition of progressive disease. In 1 patient, a new lesion appeared, and according to RECIST1.1, this is classified as progressive disease. However, since the total sum of the target lesions did not exceed a 20% increase from nadir, irRECIST classifies this as stable disease. For comparison, a recent phase II trial reported 45% disease control rate when using RECIST 1.1 [
8]. Due to the low number of patients, it is questionable if there are any real differences in response rates between the 2 studies.
Disease control rate has been shown to correlate well with OS for several cancer forms, but the role is less clear in case of platinum resistant ovarian cancer treated with PD-1 inhibitors [
13]. In our study, the disease control rate is associated with a large CI (95% CI=19–87), indicating uncertainty of the true value. The objective response rate, being 0% in our study, is far more conservative than disease control rate (44%) when evaluating treatment response. Objective response rate is defined as the proportion of patients with complete- or partial response, while disease control rate is defined as the proportion with complete- partial- and stable disease. For that reason, the objective response rate may falsely reduce the effect of a drug, while disease control rate may falsely exaggerate the effect. Both parameters should, however, be interpreted with great caution in absence of randomized trials.
The overall PFS in our study was 15 weeks. Hamanishi et al. [
8] essentially reported identical results, as PFS was 3.5 months. PFS is one of the most commonly used surrogate markers for OS in cases of first-line treatment, but the role is less clear in cases of second- and third-line treatment [
14]. To our knowledge, there is no consensus regarding the optimal use of surrogate markers in cases of platinum resistant ovarian cancer [
15]. In our study, the median OS was 30 weeks (–7 months), while Hamanishi et al. [
8] demonstrated it to be 20 months (–80 weeks). Although the cohorts were similar with respect to previous treatment lines, 1 reason for the large difference in survival may be because they included healthier patients, as 90% were ECOG 0, while 90% of our patients were ECOG 1. Another major explanatory factor may be a difference in duration of platinum resistant disease prior to PD-1 inhibitor treatment. In our study, this period was 13 months, while it is unknown in that study. A long duration of platinum resistant disease indicates that patients have more advanced disease, and consequently, they are closer to a natural end.
The median OS for patients with platinum resistant disease receiving standard chemotherapy is typically around 12–14 months [
516]. This includes survival for the entire period with platinum resistant disease and all treatment lines. Our study suggests that every additional month of platinum resistant disease is associated with 8% reduced survival (HR=1.08; p=0.004). This suggests that if PD-1 inhibitors are to be considered, 1 should start as soon as possible after platinum-resistant disease is identified. No other studies are available for comparison, and this finding should be explored in larger prospective trials.
Although not assessed in this study, the oncologist reports that clinical disease progression was usually diagnosed due to a combination of radiological worsening and increasing CA-125. The clinical symptoms, on the other hand, remained typically stable. The reason for this is not obvious, but the same discovery was also seen in a follow-up study by Hamanishi et al. [
9]. It is generally accepted that check-point inhibitors are sometimes associated with atypical response, such as pseudoprogression [
1718], which consequently may falsely be considered as progression, with subsequent termination of treatment. This is a why a confirmatory CT is recommended minimum 4 weeks after the first irPD assessment. In our study, we did not perform a confirmatory CT scans in all patients with progression since all costs were covered by the patients. The risk of pseudoprogression is also the main reason why irRECIST is thought to be more accurate than RECIST 1.1 in case of immunotherapy. The use of CA-125 in patients with platinum resistant disease is also unclear. Due to uncertainty regarding the radiological and biochemical interpretation of these patients, it is possible that we falsely classified patients as having disease progression.
The rate of grade 2 and 3 adverse events in our study was 22% (
Table 2), while no cases of grade 4 were seen. Two patients with grade 3 events had to stop treatment permanently. For comparison, Hamanishi et. al. [
8] reported grade 3–4 adverse events occurring in 8 out of 20 (40%), but only 1 patient had to permanently stop treatment. The reason for a higher rate of grade 3–4 events in that study may be coincidental, as sample sizes are small. Other studies have shown 4%–6% grade 3–4 adverse events when using PD-1 inhibitors [
1920]. These studies are larger, and consist of several different patient groups, making it difficult to compare results. Still, PD-1 inhibitor has been found to be associated with less side effects compared to standard treatment [
11]. Added toxicity has been suggested when using bevacizumab on heavily pre-treated patients [
21]. Although not statistically significant, there was a tendency towards increased toxicity in our study (OR=3.4; 95% CI=0.4–28.0; p=0.266). Further, in our study, 1 out of 7 (14%) patients receiving bevacizumab developed lethal intestinal perforation, while others have reported up to 11% perforation rate [
21]. The small number of patients and events makes it difficult to compare perforation rates among studies. Intestinal perforation is also a well-known cause of death in patients with ovarian cancer with peritoneal carcinomatosis. Although we chose to classify the perforation as a result of bevacizumab, and not PD-1 treatment, the complexity makes it difficult to conclude the true cause. We did not systematically report grade 1 events since these adverse events usually do not require medical intervention. This makes it problematic to compare the overall rate of adverse events to the results of well-designed prospective trials.
The most important limitations of this study were caused by the retrospective design, small sample size, and short observation time. Further, we do not have detailed knowledge regarding previous surgery, specific treatment lines, and there was a lack of detailed data on the treatment results. This makes it difficult to compare our results to other studies. We included a heterogeneous cohort consisting of many long-term survivors pursuing experimental treatment as a last attempt. These patients are likely to reduce efficacy of treatment as they are closer to a natural end. Likewise, patients pursuing PD-1 treatment early in the course will possibly exaggerate the effect of treatment. The methodology for measuring effect is also a limiting factor in this study since we primarily used irRECIST while previous studies have used RECIST 1.1. Small alterations in tumor volumes are susceptible to interobservational bias when deciding whether there has been tumor progression or not.
In conclusion, this study shows few adverse events, and promising clinical efficacy when using PD-1 inhibition for ovarian cancer. Prospective randomized trials are needed to confirm a better effect compared to standard treatment.








 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download