Abstract
Objective
In this study, we aimed to evaluate the clinicopathological features, obstetric, and oncological outcomes of patients diagnosed with a uterine smooth muscle tumors of uncertain malignant potential (STUMP).
Methods
A dual-institutional, database review was carried out to screen patients with STUMP who were treated with upfront surgery between January 2006 and December 2017. Data including age at the time of diagnosis, recurrence rate, disease-free survival, overall survival, and fertility outcomes were retrospectively analyzed.
Results
Fifty-seven patients with STUMPs were included in the study. The median age at the time of diagnosis was 42 (range, 16 to 75) years. The median follow-up was 57 (range, 16 to 125) months. Eight patients (14%) had recurrence during follow-up. Recurrent STUMPs were seen in seven patients and leiomyosarcoma after 14 months in one patient. Seven patients with a recurrent STUMP survived, while the remaining patient died. Recurrence rates were similar for women who underwent myomectomy and those who underwent hysterectomy. The presence of uterine localization of tumor (subserosal vs intramural-submucosal) statistically significantly affected recurrence rates (odds ratio=5.72; 95% confidence interval=1.349–24.290; p=0.018). Ten of 27 patients who underwent myomectomy for uterine myoma had fertility desire. Seven pregnancies were recorded.
Go to : 
References
1. Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol. 1994; 18:535–58.
2. Amant F, Moerman P, Vergote I. Report of an unusual problematic uterine smooth muscle neoplasm, emphasizing the prognostic importance of coagulative tumor cell necrosis. Int J Gynecol Cancer. 2005; 15:1210–2.

3. Guntupalli SR, Ramirez PT, Anderson ML, Milam MR, Bodurka DC, Malpica A. Uterine smooth muscle tumor of uncertain malignant potential: a retrospective analysis. Gynecol Oncol. 2009; 113:324–6.

4. Kalogiannidis I, Stavrakis T, Dagklis T, Petousis S, Nikolaidou C, Venizelos I, et al. A clinicopathological study of atypical leiomyomas: benign variant leiomyoma or smooth-muscle tumor of uncertain malignant potential. Oncol Lett. 2016; 11:1425–8.

5. Mowers EL, Skinner B, McLean K, Reynolds RK. Effects of morcellation of uterine smooth muscle tumor of uncertain malignant potential and endometrial stromal sarcoma: case series and recommendations for clinical practice. J Minim Invasive Gynecol. 2015; 22:601–6.

6. Ip PP, Cheung AN, Clement PB. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): a clinicopathologic analysis of 16 cases. Am J Surg Pathol. 2009; 33:992–1005.
7. Shapiro A, Ferenczy A, Turcotte R, Bruchim I, Gotlieb WH. Uterine smooth-muscle tumor of uncertain malignant potential metastasizing to the humerus as a high-grade leiomyosarcoma. Gynecol Oncol. 2004; 94:818–20.

8. Ng JS, Han A, Chew SH, Low J. A clinicopathologic study of uterine smooth muscle tumours of uncertain malignant potential (STUMP). Ann Acad Med Singapore. 2010; 39:625–8.
9. Croce S, Young RH, Oliva E. Uterine leiomyomas with bizarre nuclei: a clinicopathologic study of 59 cases. Am J Surg Pathol. 2014; 38:1330–9.
10. Dall'Asta A, Gizzo S, Musarò A, Quaranta M, Noventa M, Migliavacca C, et al. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): pathology, follow-up and recurrence. Int J Clin Exp Pathol. 2014; 7:8136–42.
11. Croce S, Ribeiro A, Brulard C, Noel JC, Amant F, Stoeckle E, et al. Uterine smooth muscle tumor analysis by comparative genomic hybridization: a useful diagnostic tool in challenging lesions. Mod Pathol. 2015; 28:1001–10.

12. Bacanakgil BH, Deveci M, Karabuk E, Soyman Z. Uterine smooth muscle tumor of uncertain malignant potential: clinicopathologic-sonographic characteristics, follow-up and recurrence. World J Oncol. 2017; 8:76–80.

13. Maltese G, Fontanella C, Lepori S, Scaffa C, Fucà G, Bogani G, et al. Atypical uterine smooth muscle tumors: a retrospective evaluation of clinical and pathologic features. Oncology. 2018; 94:1–6.

14. Basaran D, Usubutun A, Salman MC, Narin MA, Boyraz G, Turkmen O, et al. The clinicopathological study of 21 cases with uterine smooth muscle tumors of uncertain malignant potential: centralized review can purify the diagnosis. Int J Gynecol Cancer. 2018; 28:233–40.
15. Soltan MM, Albasry AM, Eldosouky MK, Abdelhamid HS. Immunoexpression of progesterone receptor, epithelial growth factor receptor and galectin-3 in uterine smooth muscle tumors. Cell Mol Biol. 2018; 64:7–12.

16. Conconi D, Chiappa V, Perego P, Redaelli S, Bovo G, Lavitrano M, et al. Potential role of BCL2 in the recurrence of uterine smooth muscle tumors of uncertain malignant potential. Oncol Rep. 2017; 37:41–7.

17. Cao HY, Yang S, Wang S, Deng LY, Lou JY. Is differential expression of p16INK4a based on the classification of uterine smooth muscle tumors associated with a different prognosis? A meta-analysis. Genet Mol Res. 2017; 16:16.

18. Hewedi IH, Radwan NA, Shash LS. Diagnostic value of progesterone receptor and p53 expression in uterine smooth muscle tumors. Diagn Pathol. 2012; 7:1.

19. O'Neill CJ, McBride HA, Connolly LE, McCluggage WG. Uterine leiomyosarcomas are characterized by high p16, p53 and MIB1 expression in comparison with usual leiomyomas, leiomyoma variants and smooth muscle tumours of uncertain malignant potential. Histopathology. 2007; 50:851–8.
20. Atkins KA, Arronte N, Darus CJ, Rice LW. The Use of p16 in enhancing the histologic classification of uterine smooth muscle tumors. Am J Surg Pathol. 2008; 32:98–102.

21. Ünver NU, Acikalin MF, Öner Ü, Ciftci E, Ozalp SS, Colak E. Differential expression of P16 and P21 in benign and malignant uterine smooth muscle tumors. Arch Gynecol Obstet. 2011; 284:483–90.

22. Croce S, Ducoulombier A, Ribeiro A, Lesluyes T, Noel JC, Amant F, et al. Genome profiling is an efficient tool to avoid the STUMP classification of uterine smooth muscle lesions: a comprehensive array-genomic hybridization analysis of 77 tumors. Mod Pathol. 2018; 31:816–28.

23. Dgani R, Piura B, Ben-Baruch G, Open M, Glezerman M, Nass D, et al. Clinical-pathological study of uterine leiomyomas with high mitotic activity. Acta Obstet Gynecol Scand. 1998; 77:74–7.

24. Vilos GA, Marks J, Ettler HC, Vilos AG, Prefontaine M, Abu-Rafea B. Uterine smooth muscle tumors of uncertain malignant potential: diagnostic challenges and therapeutic dilemmas. Report of 2 cases and review of the literature. J Minim Invasive Gynecol. 2012; 19:288–95.

25. Takeda A, Imoto S, Mori M, Nakamura H. Successful pregnancy outcome after laparoscopic-assisted excision of a bizarre leiomyoma: a case report. J Med Case Reports. 2011; 5:344.

26. Campbell JE, Knudtson JF, Valente PT, Robinson RD, Kost ER. Successful pregnancy following myomectomy for uterine smooth muscle tumor of uncertain malignant potential: a case report and review of the literature. Gynecol Oncol Rep. 2015; 15:1–3.

Go to : 
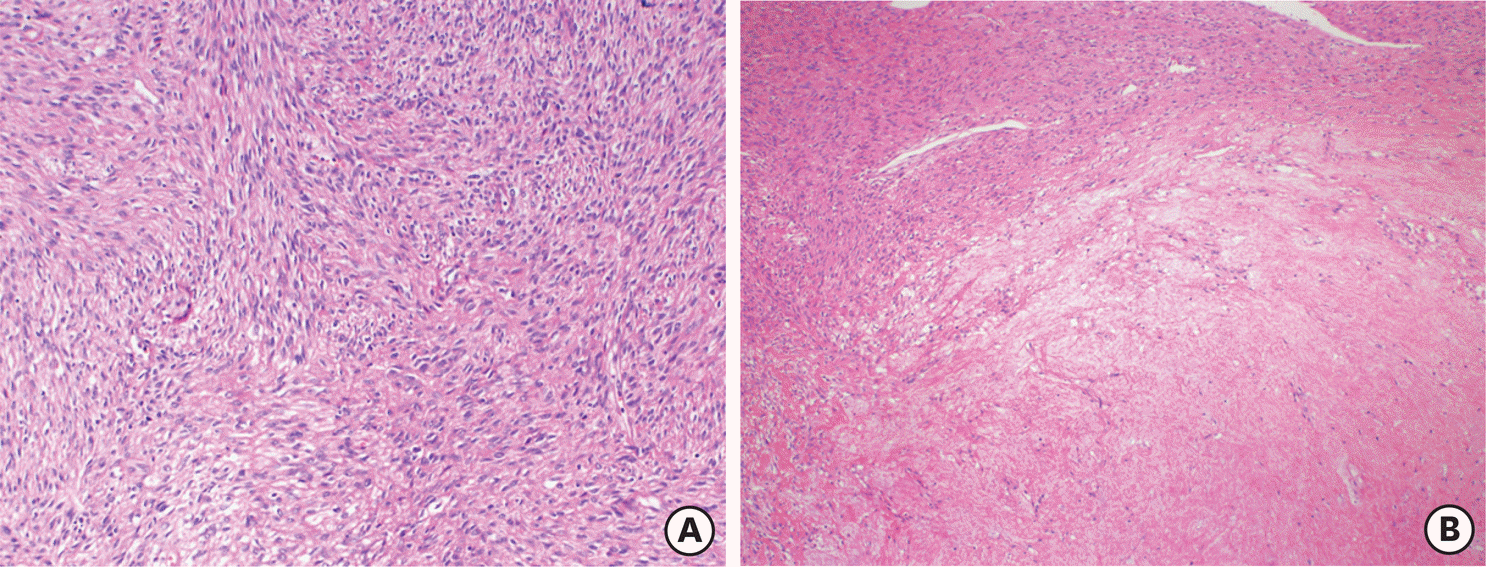 | Fig. 1.A smooth muscle tumors of uncertain malignant potential case with recurrent leiomyosarcoma. A cellular smooth muscle tumor with mild atypia (A) and necrosis of uncertain type (B) (Hematoxylin and eosin stain, ×40). |
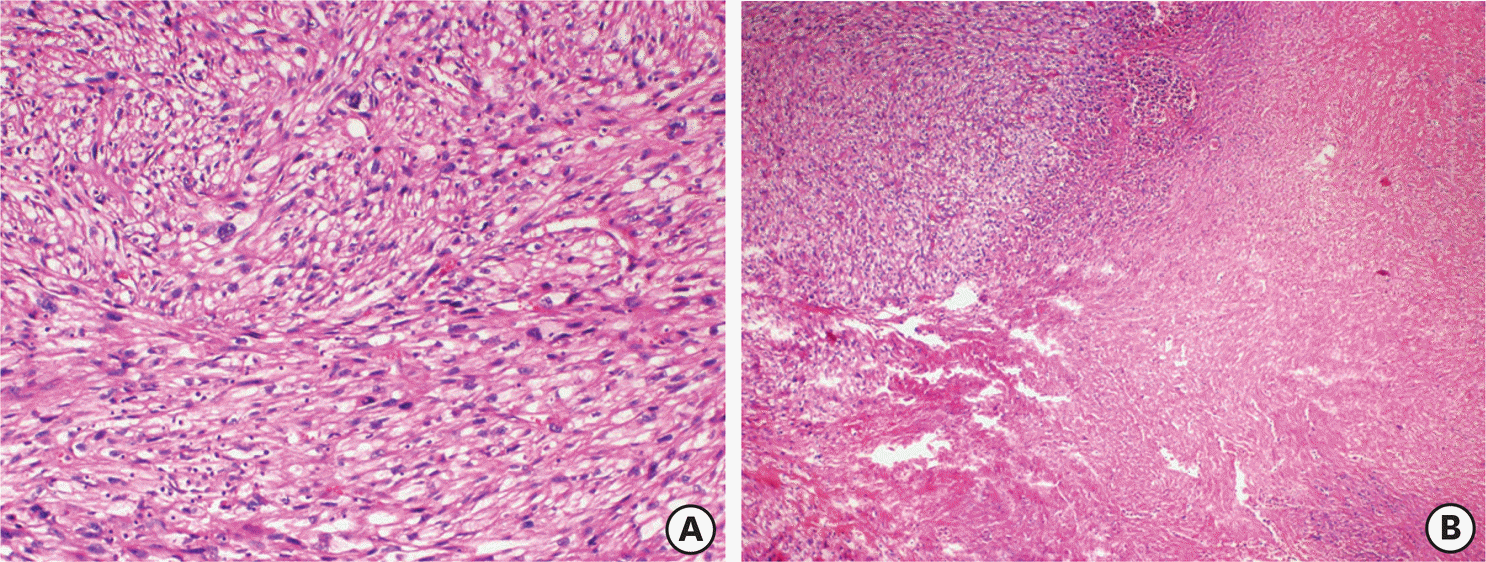 | Fig. 2.Recurrent tumor as leiomyosarcoma with tumor cell necrosis (A) (H&E ×40) and severe atypia (B) (H&E ×200). H&E, hematoxylin and eosin stain. |
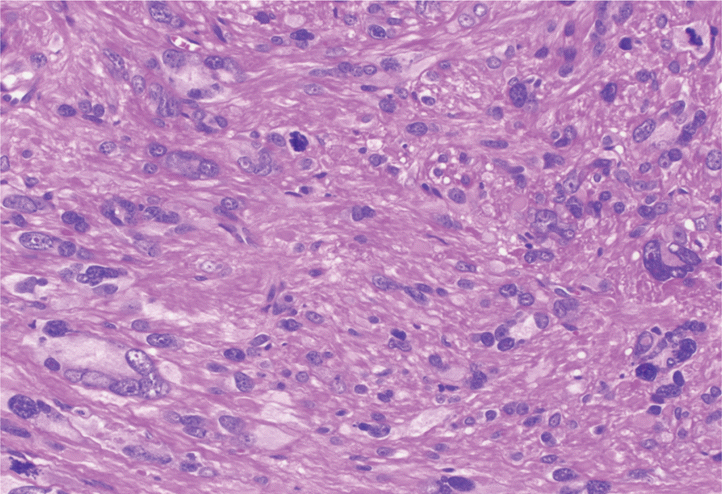 | Fig. 3.An atypical smooth muscle tumor containing bizarre cells with hyperchromatic nuclei. Scattered mitotic figures and karyorrhectic nuclei were also seen (hematoxylin and eosin stain, ×200). |
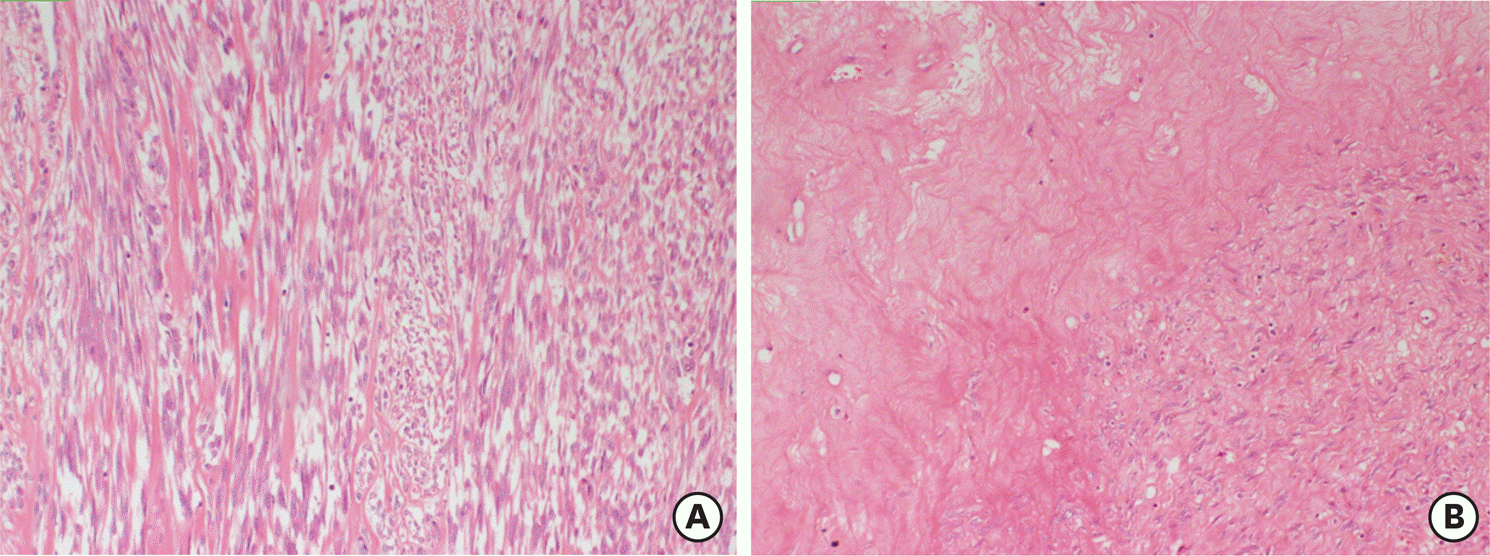 | Fig. 4.A smooth muscle tumor with mild atypia (A) and necrosis of infarct-type (B) (hematoxylin and eosin stain, ×100). |
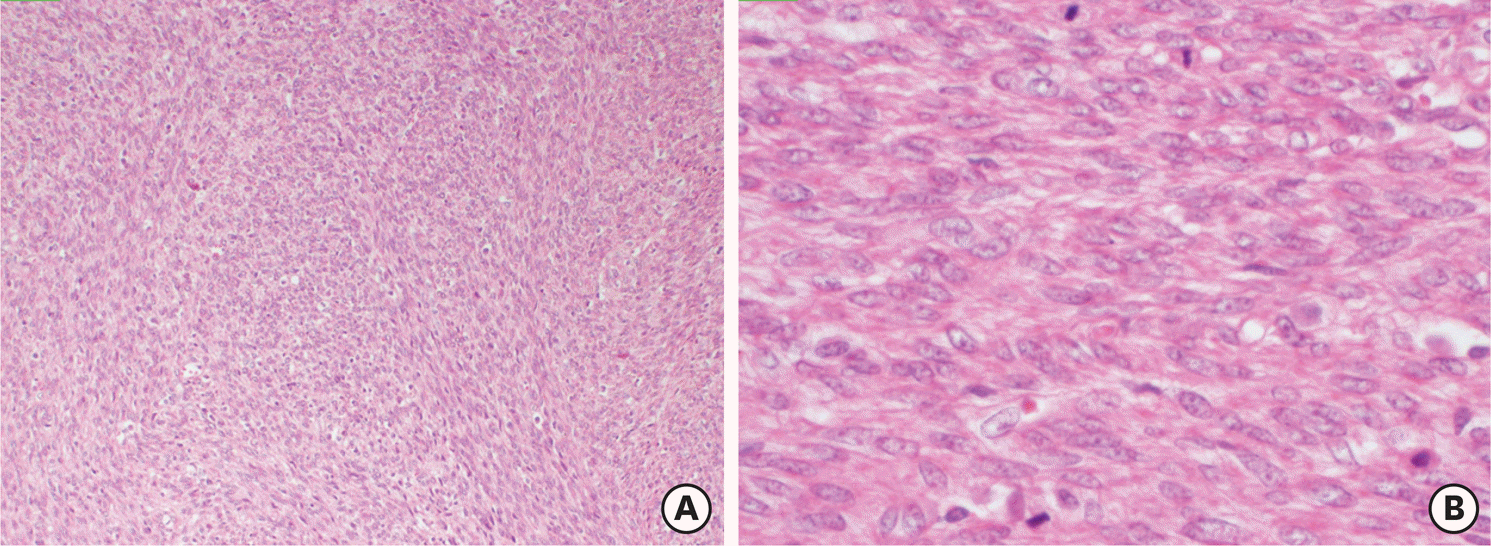 | Fig. 5.A cellular smooth muscle tumor (A) (H&E ×100) with brisk mitotic activity (B) (H&E ×400). H&E, hematoxylin and eosin stain. |
Table 1.
Demographic and clinicopathological characteristics of patients (n=57)
Table 2.
Differences between myomectomy and hysterectomy groups (n=57)
Table 3.
Clinical and pathological characteristics and outcome of patients with recurrence disease (n=8)
STUMP, uterine smooth muscle tumors of uncertain malignant potential; TAH+BSO, total abdominal hysterectomy+bilateral salpingo-oophorectomy; TAH+USO, total abdominal hysterectomy+unilateral salpingo-oophorectomy; ANED, alive with no evidence of disease; RP, retroperitoneal; CT, chemotherapy; DOD, dead of disease; LMS, leiomyosarcoma.
Table 4.
Differences between non-recurrence and recurrence groups (n=57)
Table 5.
Univariate analysis of recurrence risk factors
Table 6.
Clinical and pathological characteristics of patients with obstetrics outcomes (n=7)




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download