Abstract
Objective
The aim of this study was to verify the effects of hormone therapy (HT) on recurrence in endometrial cancer (EC) survivors using the Korean Health Insurance Review and Assessment Service (HIRA) database.
Methods
Using the HIRA claims database, we identified all Korean women who were newly diagnosed with EC and underwent surgical staging between 2010 and 2013. Patient characteristics such as age, HT exposure, lymphadenectomy, and adjuvant therapy were evaluated. Cox proportional hazards models were used to estimate the adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the recurrence of EC.
Results
The mean follow-up time of all 5,667 eligible patients was 47.5 months. Of these, 847 (14.9%) received HT. Recurrence was seen in 446 (7.8%) patients. Univariate analysis revealed an increased recurrence rate in patients older than 50 years (HR=2.05; 95% CI=1.62–2.63), patients with high-risk EC (HR=24.51; 95% CI=18.63–32.35), and patients who underwent lymphadenectomy (HR=1.52; 95% CI=1.21–2.03), and a reduced recurrence rate in patients who received HT (HR=0.62; 95% CI=0.46–0.83). Multivariate analysis confirmed the significant increase in recurrence in patients older than 50 years (HR=1.47; 95% CI=1.14–1.89) and in patients with high-risk EC (HR=23.90; 95% CI=18.12–31.51). HT did not increase the recurrence rate of EC (HR=0.81; 95% CI=0.31–2.10).
Go to : 
References
1. Lim MC, Moon EK, Shin A, Jung KW, Won YJ, Seo SS, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea, 1999–2010. J Gynecol Oncol. 2013; 24:298–302.

2. Jung KW, Won YJ, Kong HJ, Lee ES. Prediction of cancer incidence and mortality in Korea, 2018. Cancer Res Treat. 2018; 50:317–23.

3. Daraï E, Gompel A, Deval B, Laplace C, Lemoine A, Labeyrie E, et al. Hormone replacement therapy after endometrial or ovarian cancer. Gynecol Obstet Fertil. 2000; 28:198–204.
4. North American Menopause Society. The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause. 2012; 19:257–71.
5. Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous estrogen and endometrial carcinoma. N Engl J Med. 1975; 293:1164–7.

6. Ziel HK, Finkle WD. Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med. 1975; 293:1167–70.

8. O'Donnell RL, Clement KM, Edmondson RJ. Hormone replacement therapy after treatment for a gynaecological malignancy. Curr Opin Obstet Gynecol. 2016; 28:32–41.
9. Sjögren LL, Mørch LS, Løkkegaard E. Hormone replacement therapy and the risk of endometrial cancer: a systematic review. Maturitas. 2016; 91:25–35.

10. Ayhan A, Taskiran C, Simsek S, Sever A. Does immediate hormone replacement therapy affect the oncologic outcome in endometrial cancer survivors? Int J Gynecol Cancer. 2006; 16:805–8.
12. Mørch LS, Kjaer SK, Keiding N, Løkkegaard E, Lidegaard Ø. The influence of hormone therapies on type I and II endometrial cancer: a nationwide cohort study. Int J Cancer. 2016; 138:1506–15.

13. Barakat RR, Bundy BN, Spirtos NM, Bell J, Mannel RS. Gynecologic Oncology Group Study. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2006; 24:587–92.

14. Lee SJ, Yeo SG, Kang SB, Park DC. Attitudes and practices of Korean gynecologists towards hormone replacement therapy in endometrial cancer survivors. Eur J Gynaecol Oncol. 2013; 34:513–7.
15. Lim S, Kim YH, Lee KB, Lee JM. The influence of hormone therapy with drospirenone-estradiol on endometrioid type endometrial cancer patients. J Gynecol Oncol. 2018; 29:e72.

16. Kim HA, Kim S, Seo YI, Choi HJ, Seong SC, Song YW, et al. The epidemiology of total knee replacement in South Korea: national registry data. Rheumatology (Oxford). 2008; 47:88–91.

17. Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine neoplasms, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018; 16:170–99.

18. Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006; 24:36–44.

19. Creasman WT, Henderson D, Hinshaw W, Clarke-Pearson DL. Estrogen replacement therapy in the patient treated for endometrial cancer. Obstet Gynecol. 1986; 67:326–30.

20. Lee RB, Burke TW, Park RC. Estrogen replacement therapy following treatment for stage I endometrial carcinoma. Gynecol Oncol. 1990; 36:189–91.

21. Baker DP. Estrogen-replacement therapy in patients with previous endometrial carcinoma. Compr Ther. 1990; 16:28–35.
22. Bryant GW. Administration of estrogens to patients with a previous diagnosis of endometrial adenocarcinoma. South Med J. 1990; 83:725–6.

23. Chapman JA, DiSaia PJ, Osann K, Roth PD, Gillotte DL, Berman ML. Estrogen replacement in surgical stage I and II endometrial cancer survivors. Am J Obstet Gynecol. 1996; 175:1195–200.

24. Suriano KA, McHale M, McLaren CE, Li KT, Re A, DiSaia PJ. Estrogen replacement therapy in endometrial cancer patients: a matched control study. Obstet Gynecol. 2001; 97:555–60.

25. Chlebowski RT, Anderson GL, Sarto GE, Haque R, Runowicz CD, Aragaki AK, et al. Continuous combined estrogen plus progestin and endometrial cancer: The Women's Health Initiative Randomized Trial. J Natl Cancer Inst. 2015; 108:djv350.

26. Shim SH, Lee SJ, Kim SN. Effects of hormone replacement therapy on the rate of recurrence in endometrial cancer survivors: a meta-analysis. Eur J Cancer. 2014; 50:1628–37.

27. Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000; 355:1404–11.
28. Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991; 40:55–65.

29. Maggi R, Lissoni A, Spina F, Melpignano M, Zola P, Favalli G, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006; 95:266–71.

30. Hogberg T, Signorelli M, de Oliveira CF, Fossati R, Lissoni AA, Sorbe B, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer–results from two randomised studies. Eur J Cancer. 2010; 46:2422–31.

31. de Boer SM, Powell ME, Mileshkin L, Katsaros D, Bessette P, Haie-Meder C, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018; 19:295–309.
32. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017; 24:728–53.
33. Seo GH, Kim JH, Ku JH. Clinical practice pattern of immediate intravesical chemotherapy following transurethral resection of a bladder tumor in Korea: National Health Insurance Database Study. Sci Rep. 2016; 6:22716.

34. Lee SU, Soh M, Ryu V, Kim CE, Park S, Roh S, et al. Analysis of the Health Insurance Review and Assessment Service data from 2011 to 2015. Int J Ment Health Syst. 2018; 12:9.
Go to : 
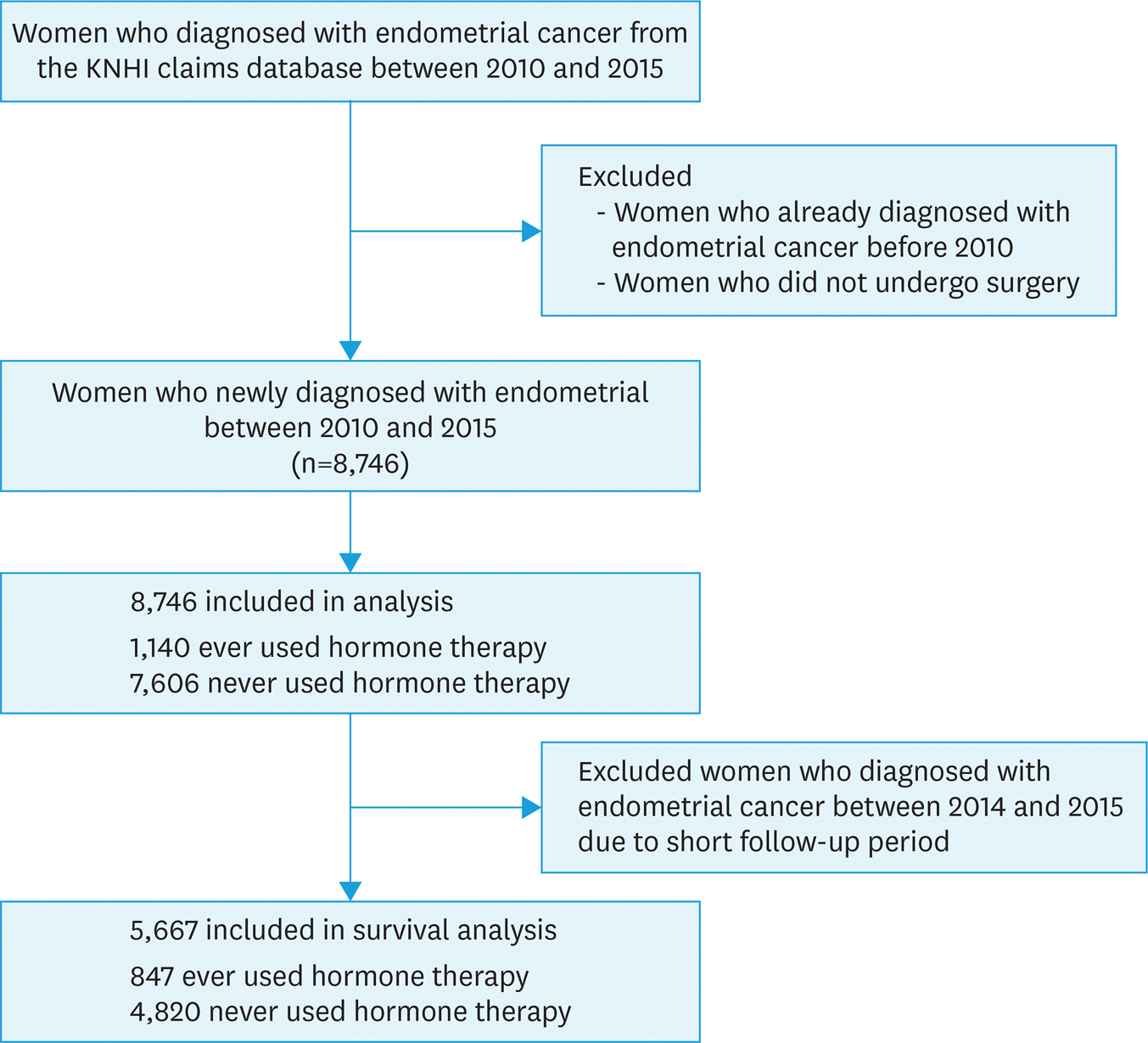 | Fig. 1.A flowchart of the study design. EC, endometrial cancer; HT, hormone therapy; KNHI, Korean National Health Insurance. |
Table 1.
Annual number of patients who newly diagnosed with endometrial cancer between 2010 and 2015 in Korea
Table 2.
Clinical characteristics of patients by use of HT (2010–2015)
Table 3.
Univariate Cox regression model for associations between clinical factors and recurrence (2010–2013)
| Characteristics | Recurrence* (n=446) | HR | 95% CI | p-value |
|---|---|---|---|---|
| Age (yr) | <0.001 | |||
| <50 | 75 (4.7) | 1.00 | ||
| ≥50 | 371 (9.1) | 2.05 | 1.62–2.63 | |
| Lymphadenectomy | 0.002 | |||
| No | 75 (5.5) | 1.00 | ||
| Yes | 379 (8.5) | 1.52 | 1.21–2.03 | |
| Adjuvant therapy | <0.001 | |||
| No adjuvant therapy+brachytherapy+EBRT (low-intermediate risk EC) | 59 (1.3) | 1.00 | ||
| Chemotherapy (high-risk EC) | 387 (31.7) | 24.51 | 18.63–32.35 | |
| Postoperative HT | 0.001 | |||
| No | 396 (8.2) | 1.00 | ||
| Yes | 50 (5.9) | 0.62 | 0.46–0.83 | |
| Type of HT | ||||
| No | 396 (8.2) | 1.00 | ||
| Estrogen | 10 (3.9) | 0.39 | 0.23–0.71 | 0.002 |
| Estrogen plus progesterone | 6 (5.4) | 0.45 | 0.29–0.98 | 0.034 |
| Tibolone | 14 (5.3) | 0.50 | 0.35–0.84 | 0.008 |
| Progesterone | 20 (9.5) | 0.89 | 0.62–1.43 | 0.588 |
Table 4.
Multivariate Cox regression model for recurrence risk of EC according to each prognostic factor
| Characteristics | HR | 95% CI | p-value |
|---|---|---|---|
| Age ≥50 yr | 1.47 | 1.14–1.89 | 0.003 |
| Assessment of lymph node | 1.07 | 0.82–1.39 | 0.365 |
| Adjuvant therapy* | 23.90 | 18.12–31.51 | <0.001 |
| Postoperative HT | 0.81 | 0.31–2.10 | 0.662 |
| Type of HT | |||
| Estrogen | 0.78 | 0.31–1.96 | 0.602 |
| Estrogen plus progesterone | 0.57 | 0.21–1.57 | 0.278 |
| Tibolone | 0.88 | 0.34–2.29 | 0.795 |
| Progesterone | 1.02 | 0.39–2.67 | 0.977 |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download