Abstract
Degenerating nodules (DNs), which primarily manifest as benign thyroid nodules, are one of the main causes of discordance in ultrasonography (US) and cytological assessments. Intranodular hemorrhage is one of the mechanisms contributing to discordant nodules, and an impaired blood supply may explain further DN shrinkage and infarction. The surgical specimens can be divided into acute and chronic stages based on the histological changes, which usually mimic the US features of malignant tumors. Serial US follow-up should be recommended instead of other unnecessary procedures. However, repeated fine-needle aspiration, diagnostic surgery, or core-needle biopsy may still be necessary for indeterminable or highly suspicious DNs.
Ultrasonography (US) is a standard tool used to evaluate thyroid nodules at follow-up assessments. These nodules may remain stable or may show changes in size at follow-up (12). Thyroid nodules that have decreased in size are usually benign and have various names, such as degenerating nodules (DNs) (34), collapsing benign cystic nodules (5), mummified thyroid syndrome (67), and vanishing tumors (89). Among these, DNs have been mainly described as benign thyroid nodules (34567101112); however, they are also related to malignant thyroid cancer (913), which can be induced spontaneously or after fine-needle aspiration (FNA) or core-needle biopsy (CNB) (345678910111213).
DNs may sometimes show suspicious US features, which may cause confusion to many doctors despite a reduction in their size. DNs that show a size reduction on follow-up US are identified as benign rather than malignant; however, these nodules may occasionally appear without any previous US findings. Furthermore, DNs frequently show suspicious US features and even FNA may reveal variable cytological results, such as benign findings, atypia of undetermined significance (14), or nondiagnostic results (1516). In such cases with US-cytology discordance (i.e., cytologically benign nodules with suspicious US features), the false-negative rate of FNA is nonnegligible (1718). The incidence of US-cytology discordant nodules is 2.8–19.7% (19), and guidelines recommend repeated US-guided FNA in such cases (202122). One of the main causes of US-cytology discordant nodules is DNs.
The histological findings for DNs show a decrease in or absence of follicular cells and secondary reactive or degenerative changes such as hemorrhage, degeneration, infarction, fibrosis, vascular thrombosis/proliferation, stromal cell proliferation, and squamous metaplasia (10). These changes include worrisome histological features such as cytologic atypia, architectural atypia, and necrosis, which often lead to issues in the cytological interpretation of FNA specimens.
These US-cytology discordant nodules may lead to unnecessary repeated biopsies or diagnostic surgical procedures. Therefore, in this review, we will discuss the mechanism and US-pathology correlations of DNs to improve their differential diagnosis and management strategies.
Studies on the natural history of benign thyroid nodules have shown that cystic or predominantly cystic nodules are more likely to decrease in size than solid nodules (123). In cystic or predominantly cystic thyroid nodules, the cystic portion can be spontaneously absorbed, desiccated, or crumpled over time. In solid nodules, iatrogenic injury by FNA/CNB can cause venous thrombosis and/or internal bleeding, which are mechanisms that potentially lead to nodule degeneration (7101124). Intranodular hemorrhage (spontaneous or FNA-induced hemorrhage) compressing peripheral nodule tissues is believed to be one of the initial mechanisms of DN development (Fig. 1). Hemorrhage usually occurs in thyroid nodules and may be associated with their rich blood supply. The prevalence of hemorrhage within thyroid nodules after biopsy is reportedly between 26.5% and 93.3% (102526), whereas even in patients who do not undergo FNA, the hemorrhage rate is still as high as 33.3% (10). An impaired blood supply due to venous thrombosis and hematoma regression may explain the further shrinkage and infarction that occurs later in the degradation process (7101124).
The considerable variation in the histological changes in DNs makes diagnosis difficult. Surgical specimens of DNs are divided into an acute stage and a chronic stage based on the interval between FNA and surgical excision of the lesion (11). LiVolsi and Merino (11) stressed the importance of histological changes in surgical specimens by proposing the “Worrisome Histologic Alterations Following Fine-Needle Aspiration of the Thyroid” (WHAFFT) syndrome. Symptoms of WHAFFT syndrome include acute changes such as hemorrhage, granulation tissue, mitosis, granulomas, capsular distortion (pseudoinvasion), and infarction and chronic changes such as fibrosis, metaplasia, infarction, cyst formation, papillary degeneration, papillary endothelial proliferation, and calcification (11). Hemorrhage and granulated tissues are the main histological changes observed within 3 weeks after FNA, which is the so-called acute stage. The chronic stage typically begins one month after FNA, during which fibrosis and capsule distortion are the dominant changes (1011).
Biopsy specimens show different gross features in FNA and CNB according to the degeneration stage. In the early stage, hemorrhage appears as a reddish toothpaste-like muddy material on FNA and CNB (Fig. 2). Cytopathological characteristics include numerous degenerated red blood cells, few hemosiderin-laden macrophages, and a few equivocal microcalcifications. In the second stage of hemorrhage regression, CNB specimens show a brown bread-like material containing diffuse stromal fibrosis with chronic inflammation and histiocyte deposition (Fig. 3). Whitish hard material can be seen on the CNB specimen until the chronic stage, at which dense stromal fibrosis with/without calcifications can be observed (Fig. 4). These cases usually show no histological evidence of malignancy, which is key to diagnosing DNs as benign lesions; however, atypical cells occasionally develop, resulting in atypia of undetermined significance on cytopathological assessments. Importantly, in the chronic stage, nondiagnostic results with FNA specimens are common because these specimens contain few follicular cells, and the consistency of DNs becomes harder due to fibrosis.
Despite the gradual size reduction of DNs, their worrisome histological alterations usually lead to US features mimicking malignancy (2728293031), upgrading their thyroid imaging reporting and data system (Korean-TIRADS) category (61032).
Lee et al. (4) reported that all cystic portions in nodules (8/8) disappeared and were replaced with solid content (Fig. 5). The degree of malignant US features (i.e., spiculated margin and marked hypoechogenicity) increased during the follow-up period (6% vs. 13% and 19% vs. 38%, respectively) (Fig. 6). Micro- and macrocalcifications also became more prevalent in the final US findings than in the initial images (19% vs. 32%) (4).
Fibrosis is closely related to hypoechogenicity on US (3272833). The relatively high incidence of intranodular fibrosis in DNs (66.0–100%) may explain the suspicious US findings (410). Calcification, which is another malignant US feature, usually develops in 17.9–25.0% of DNs within 2 months after FNA (3410). However, colloid material can mimic calcifications after degeneration. More importantly, considering the similar incidences of the appearance of microcalcifications (p = 0.734) and macrocalcifications (p = 0.538) in DN and papillary thyroid carcinoma (PTC) (5), the presence of calcifications is problematic. Collapse (428) and capsular distortion (pseudoinvasion) may contribute to ill-defined margins (10). Capsular distortion was found in 7.6–14% of nodules after FNA (10). Similarly, the taller-than-wide shape of DNs correlates with asymmetric fibrous traction, especially transverse shrinking (7).
Several studies have tried to differentiate DNs from thyroid carcinoma on US. Significant differences were found between DNs and PTC in shape (ovoid-to-round vs. taller-than-wide), margins (ill-defined vs. spiculated), low-echoic halo, and inner isoechoic rim (5343536373839); however, these findings overlap in both diseases.
Among these US features, inner isoechoic rim and low-echoic halo showed the highest diagnostic accuracies with acceptable negative predictive values (5). An inner isoechoic rim was defined as a thin continuous isoechoic rim along more than half of the inner margin of a nodule, which may correspond with the intact solid wall of preexisting cystic nodules (5). Interestingly, a tumor capsule or a fibrotic pseudocapsule that is compressed in normal thyroid tissue or chronic inflammatory infiltrates can manifest as a low-echoic halo (3040), which is defined as a hypoechoic rim surrounding the outer margin of a nodule.
Posterior US shadowing was also reported as a useful US feature for a correct final diagnosis; this shadowing is associated with an uneven interface due to shrinkage encapsulation or peripheral fibrosis (3041). However, the lack of vasculature does not definitively distinguish DNs from PTC because a strong stromal desmoid reaction can suppress angiogenesis (42).
Serial US images and findings, including thyroid nodule volume, echotexture, and echogenicity obtained from a comparison with previous US results, may warrant US follow-up instead of an unnecessary repeated biopsy or diagnostic surgery (671043).
However, genuine PTCs rarely exhibit volume stability and can even shrink over time (44). In addition to these rare cases, when the patient's history is unavailable, biopsy should be recommended.
FNA is an effective and accurate technique for diagnosing thyroid nodules. However, DNs tend to present benign results, atypia of undetermined significance, or nondiagnostic results upon FNA because of the histological nature of scarce follicular cells and hard fibrosis. Moreover, US-cytology discordant nodules, defined by benign cytological findings but suspiciously malignant US findings, have a markedly increased risk of malignancy of 13.6–56.6% (102021314546). Given the above conditions, even if the FNA results are benign, repeat FNA or diagnostic surgery may still be necessary due to the malignant US features.
Recent studies seem to support the assumption that CNB is more useful than repeat FNA for avoiding inappropriate diagnostic surgery in patients with initial nondiagnostic results (1516) or atypia of undetermined significance (4748) and discordant US-cytology results (3). CNB of solid nodules yields a high level of diagnostic accuracy (97–98%) and has a lower rate of inconclusive or nondiagnostic results (3.2–7.2%) than FNA (349505152). Yeon et al. (15) revealed that diagnostic surgery can be prevented by CNB in 96% of patients with two series of nondiagnostic FNA results.
The correlation between pathologic characteristics and US findings for DNs may explain the utility of CNB for diagnosis (4). There are relatively few follicular cells in the center of a DN, whereas plenty of follicular cells are located adjacent to the peripheral area (Fig. 7) (4). Utilizing the central region as a target may increase the nondiagnostic risk of FNA. In contrast, CNB specimens containing both the center and peripheral regions may offer significantly enhanced diagnostic value (4). Therefore, when a CNB specimen shows fibrosis and hemorrhage with scarce follicular cells in the corresponding suspicious area within DNs, such a nodule can be more confidently considered to be a benign lesion (Fig. 8).
The risk of malignant discordant US-CNB nodules was lower (3.6–5.5%) than that of malignant discordant US-FNA nodules (13.6–56.6%) (29). This finding suggests that rebiopsy or diagnostic surgery for US-CNB discordant thyroid nodules should be carefully considered. Caution should be taken because CNB may mistarget the diagnostic portion if it is limited to one or two CNB sessions (29); malignant nodules may also spontaneously degenerate.
Most DNs are benign, but they frequently show suspicious US features. An understanding of the mechanism, US findings, pathological findings, and serial changes in DNs can minimize unnecessary procedures for patients. Some US features, including ovoid-to-round shape, ill-defined borders, low-echoic halo, inner isoechoic rim, and posterior shadowing, may help diagnose DNs. Follow-up US should be recommended first in cases showing nodule shrinkage and changes in echotexture and echogenicity on serial US images. For patients whose history is unavailable or whose nodules remain highly suspicious, biopsy should also be recommended. FNA may be the first choice, but DNs tend to present benign results, atypia of undetermined significance, or nondiagnostic results in FNA because of the histological nature of scarce follicular cells and hard fibrosis. Given the above conditions, it would be more useful to perform CNB instead of repeat FNA to avoid unnecessary diagnostic surgery.
References
1. Alexander EK, Hurwitz S, Heering JP, Benson CB, Frates MC, Doubilet PM, et al. Natural history of benign solid and cystic thyroid nodules. Ann Intern Med. 2003; 138:315–318. PMID: 12585829.

2. Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, et al. AACE/AME/ETA Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. J Endocrinol Invest. 2010; 33(5 Suppl):1–50.

3. Ha EJ, Baek JH, Lee JH, Song DE, Kim JK, Shong YK, et al. Sonographically suspicious thyroid nodules with initially benign cytologic results: the role of a core needle biopsy. Thyroid. 2013; 23:703–708. PMID: 23544697.

4. Lee HY, Baek JH, Ha EJ, Park JW, Lee JH, Song DE, et al. Malignant-looking thyroid nodules with size reduction: core needle biopsy results. Ultrasonography. 2016; 35:327–334. PMID: 27184652.

5. Ko MS, Jeong KS, Shong YK, Gong GY, Baek JH, Lee JH. Collapsing benign cystic nodules of the thyroid gland: sonographic differentiation from papillary thyroid carcinoma. AJNR Am J Neuroradiol. 2012; 33:124–127. PMID: 22158923.

6. Lacout A, Chevenet C, Marcy PY. Suspicious thyroid nodule management with nondiagnostic results at cytologic examination: how to diagnosis mummified benign thyroid nodules. Radiology. 2015; 277:303–304. PMID: 26402498.

7. Lacout A, Chevenet C, Marcy PY. Mummified thyroid syndrome. AJR Am J Roentgenol. 2016; 206:837–845. PMID: 27003052.

8. Kholová I. Vanishing thyroid gland tumors: infarction as consequence of FNA? Diagn Cytopathol. 2016; 44:568–573. PMID: 27094979.

9. Bhatia P, Deniwar A, Mohamed HE, Sholl A, Murad F, Aslam R, et al. Vanishing tumors of thyroid: histological variations after fine needle aspiration. Gland Surg. 2016; 5:270–277. PMID: 27294033.

10. Bolat F, Kayaselcuk F, Nursal TZ, Reyhan M, Bal N, Yildirim S, et al. Histopathological changes in thyroid tissue after fine needle aspiration biopsy. Pathol Res Pract. 2007; 203:641–645. PMID: 17582696.

11. LiVolsi VA, Merino MJ. Worrisome histologic alterations following fine-needle aspiration of the thyroid (WHAFFT). Pathol Annu. 1994; 29(Pt 2):99–120. PMID: 7936753.
12. Polyzos SA, Patsiaoura K, Zachou K. Histological alterations following thyroid fine needle biopsy: a systematic review. Diagn Cytopathol. 2009; 37:455–465. PMID: 19306421.

13. Lacout A, Isaac S, Marcy PY. Micro-medullary thyroid carcinoma: a diagnosis not to be missed. Postgrad Med J. 2015; 91:236–237. PMID: 25862709.

14. Cochand-Priollet B, Vielh P, Royer B, Belleannée G, Collet JF, Goubin-Versini I, et al. sous l'égide de la Société française de cytologie clinique. [Thyroid cytopathology: Bethesda System 2010]. Ann Pathol. 2012; 32:177–183. PMID: 22748331.
15. Yeon JS, Baek JH, Lim HK, Ha EJ, Kim JK, Song DE, et al. Thyroid nodules with initially nondiagnostic cytologic results: the role of core-needle biopsy. Radiology. 2013; 268:274–280. PMID: 23525204.

16. Screaton NJ, Berman LH, Grant JW. US-guided core-needle biopsy of the thyroid gland. Radiology. 2003; 226:827–832. PMID: 12601219.

17. Gharib H, Goellner JR. Fine-needle aspiration biopsy of the thyroid: an appraisal. Ann Intern Med. 1993; 118:282–289. PMID: 8420446.

18. Hamburger JI, Hamburger SW. Fine needle biopsy of thyroid nodules: avoiding the pitfalls. N Y State J Med. 1986; 86:241–249. PMID: 3520410.
19. Ito Y, Amino N, Yokozawa T, Ota H, Ohshita M, Murata N, et al. Ultrasonographic evaluation of thyroid nodules in 900 patients: comparison among ultrasonographic, cytological, and histological findings. Thyroid. 2007; 17:1269–1276. PMID: 17988196.

20. Kwak JY, Koo H, Youk JH, Kim MJ, Moon HJ, Son EJ, et al. Value of US correlation of a thyroid nodule with initially benign cytologic results. Radiology. 2010; 254:292–300. PMID: 20019136.

21. Kwak JY, Kim EK, Kim HJ, Kim MJ, Son EJ, Moon HJ. How to combine ultrasound and cytological information in decision making about thyroid nodules. Eur Radiol. 2009; 19:1923–1931. PMID: 19277669.

22. Chernyavsky VS, Shanker BA, Davidov T, Crystal JS, Eng O, Ibrahim K, et al. Is one benign fine needle aspiration enough? Ann Surg Oncol. 2012; 19:1472–1476. PMID: 21969084.

23. Kuma K, Matsuzuka F, Kobayashi A, Hirai K, Morita S, Miyauchi A, et al. Outcome of long standing solitary thyroid nodules. World J Surg. 1992; 16:583–587. discussion 587-588. PMID: 1413828.

24. Aulicino MR, Kaneko M, Uinger PD. Excessive endothelial cell proliferation occurring in an organizing thyroid hematoma: report of a case and review of the literature. Endocr Pathol. 1995; 6:153–158. PMID: 12114651.

25. Ersöz C, Soylu L, Erkoçak EU, Tetiker T, Gümürdülü D. Histologic alterations in the thyroid gland after fine-needle aspiration. Diagn Cytopathol. 1997; 16:230–232. PMID: 9099543.

26. Pandit AA, Phulpagar MD. Worrisome histologic alterations following fine needle aspiration of the thyroid. Acta Cytol. 2001; 45:173–179. PMID: 11284302.

27. Ha EJ, Baek JH, Lee JH, Lee HY, Song DE, Kim JK, et al. A focal marked hypoechogenicity within an isoechoic thyroid nodule: is it a focal malignancy or not? Acta Radiol. 2015; 56:814–819. PMID: 24938659.

28. Jung SL, Jung CK, Kim SH, Kang BJ, Ahn KJ, Kim BS, et al. Histopathologic findings related to the indeterminate or inadequate results of fine-needle aspiration biopsy and correlation with ultrasonographic findings in papillary thyroid carcinomas. Korean J Radiol. 2010; 11:141–148. PMID: 20191060.

29. Chung SR, Baek JH, Park HS, Choi YJ, Sung TY, Song DE, et al. Ultrasound-pathology discordant nodules on core-needle biopsy: malignancy risk and management strategy. Thyroid. 2017; 27:707–713. PMID: 28326900.

30. Koo JH, Shin JH, Han BK, Ko EY, Kang SS. Cystic thyroid nodules after aspiration mimicking malignancy: sonographic characteristics. J Ultrasound Med. 2010; 29:1415–1421. PMID: 20876894.
31. Moon HJ, Kim EK, Kwak JY. Malignancy risk stratification in thyroid nodules with benign results on cytology: combination of thyroid imaging reporting and data system and Bethesda system. Ann Surg Oncol. 2014; 21:1898–1903. PMID: 24558069.

32. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Korean Society of Thyroid Radiology (KSThR) and Korean Society of Radiology. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2016; 17:370–395. PMID: 27134526.

33. Kim JH, Na DG, Lee H. Ultrasonographic echogenicity and histopathologic correlation of thyroid nodules in core needle biopsy specimens. Korean J Radiol. 2018; 19:673–681. PMID: 29962873.

34. Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002; 87:1941–1946. PMID: 11994321.

35. Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002; 178:687–691. PMID: 11856699.

36. Frates MC, Benson CB, Doubilet PM, Cibas ES, Marqusee E. Can color Doppler sonography aid in the prediction of malignancy of thyroid nodules? J Ultrasound Med. 2003; 22:127–131. quiz 132–134. PMID: 12562117.

37. Iannuccilli JD, Cronan JJ, Monchik JM. Risk for malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J Ultrasound Med. 2004; 23:1455–1464. PMID: 15498910.
38. Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Thyroid Study Group, Korean Society of Neuro- and Head and Neck Radiology. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology. 2008; 247:762–770. PMID: 18403624.

39. Silver RJ, Parangi S. Management of thyroid incidentalomas. Surg Clin North Am. 2004; 84:907–919. PMID: 15145242.

40. Propper RA, Skolnick ML, Weinstein BJ, Dekker A. The nonspecificity of the thyroid halo sign. J Clin Ultrasound. 1980; 8:129–132. PMID: 6767745.

41. Sillery JC, Reading CC, Charboneau JW, Henrichsen TL, Hay ID, Mandrekar JN. Thyroid follicular carcinoma: sonographic features of 50 cases. AJR Am J Roentgenol. 2010; 194:44–54. PMID: 20028904.
42. Lacout A, Marcy PY. Highlights on power Doppler US of thyroid malignancy. Radiology. 2010; 257:586–587. author reply 587. PMID: 20959551.

43. Lacout A, Chevenet C, Marcy PY. Reverse mummified thyroid syndrome. AJR Am J Roentgenol. 2016; 207:W23. PMID: 27145041.

44. Simpson KW, Albores-Saavedra J. Unusual findings in papillary thyroid microcarcinoma suggesting partial regression: a study of two cases. Ann Diagn Pathol. 2007; 11:97–102. PMID: 17349567.

45. Hwang SH, Sung JM, Kim EK, Moon HJ, Kwak JY. Imaging-cytology correlation of thyroid nodules with initially benign cytology. Int J Endocrinol. 2014; 2014:491508. PMID: 25374600.

46. Shin JH, Han BK, Ko K, Choe YH, Oh YL. Value of repeat ultrasound-guided fine-needle aspiration in nodules with benign cytological diagnosis. Acta Radiol. 2006; 47:469–473. PMID: 16796308.

47. Nasrollah N, Trimboli P, Guidobaldi L, Cicciarella Modica DD, Ventura C, Ramacciato G, et al. Thin core biopsy should help to discriminate thyroid nodules cytologically classified as indeterminate. A new sampling technique. Endocrine. 2013; 43:659–665. PMID: 23070753.

48. Park KT, Ahn SH, Mo JH, Park YJ, Park DJ, Choi SI, et al. Role of core needle biopsy and ultrasonographic finding in management of indeterminate thyroid nodules. Head Neck. 2011; 33:160–165. PMID: 20848434.

49. Suh CH, Baek JH, Lee JH, Choi YJ, Kim JK, Sung TY, et al. The role of core-needle biopsy as a first-line diagnostic tool for initially detected thyroid nodules. Thyroid. 2016; 26:395–403. PMID: 26651390.

50. Yoon JH, Kim EK, Kwak JY, Moon HJ. Effectiveness and limitations of core needle biopsy in the diagnosis of thyroid nodules: review of current literature. J Pathol Transl Med. 2015; 49:230–235. PMID: 26018514.

51. Zhang M, Zhang Y, Fu S, Lv F, Tang J. Thyroid nodules with suspicious ultrasound findings: the role of ultrasound-guided core needle biopsy. Clin Imaging. 2014; 38:434–438. PMID: 24746446.

52. Trimboli P, Nasrollah N, Guidobaldi L, Taccogna S, Cicciarella Modica DD, Amendola S, et al. The use of core needle biopsy as first-line in diagnosis of thyroid nodules reduces false negative and inconclusive data reported by fine-needle aspiration. World J Surg Oncol. 2014; 12:61. PMID: 24661377.

Fig. 1
US features of degenerating nodule.
Cystic portion is decreased and solid isoechoic portion (arrow, A) shows degeneration as isoechoic rim (arrow, B). US = ultrasonography
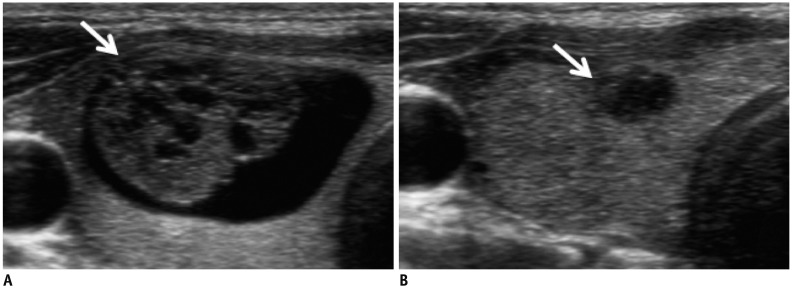
Fig. 2
Early-stage degenerating thyroid nodule.
US image shows markedly hypoechoic solid nodule (A). Early stage of hemorrhage shows reddish gel-like material on FNA (B) and CNB (C). Consistency is like toothpaste (D). CNB = core-needle biopsy, FNA = fine-needle aspiration
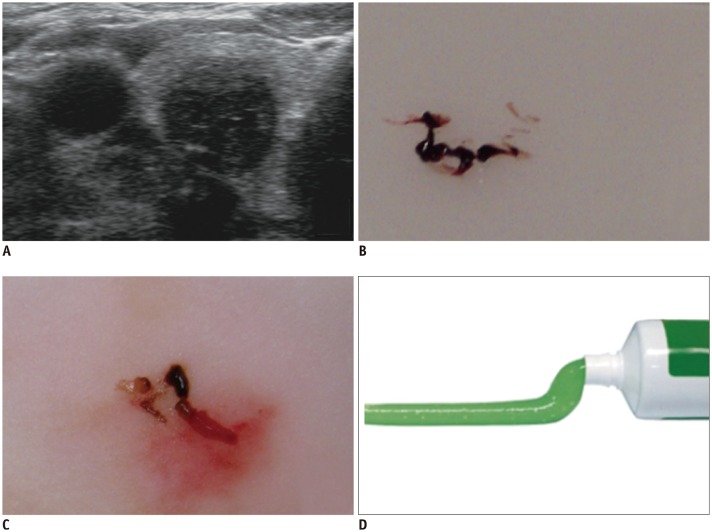
Fig. 3
Second-stage degenerating thyroid nodule.
US features of CNB (A) and CNB specimen (B) show brown semi-solid (bread-like) material (C) containing diffuse stromal fibrosis with chronic inflammation and deposition of histiocytes.
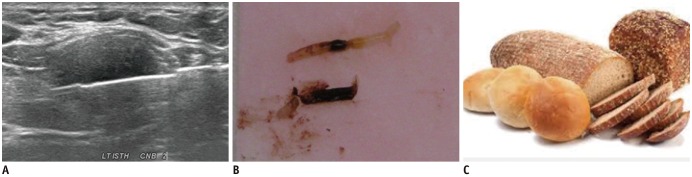
Fig. 4
Chronic-stage degenerating thyroid nodule.
A. US image shows hypoechoic solid nodule with hyperechoic strip and acoustic shadow. B. Pathologic specimen shows dense stromal fibrosis with/without calcifications (HE, × 40). C. CNB shows whitish hard material. HE = hematoxylin and eosin stain
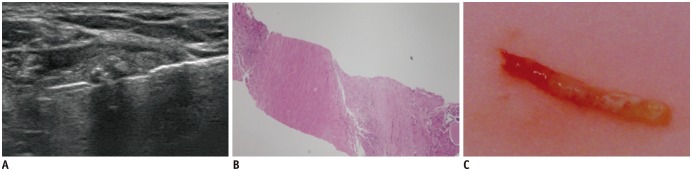
Fig. 5
Degeneration of cystic thyroid nodule.
A. US image obtained in December 2007 showed that nodule was colloid cyst. B. In December 2010, nodule significantly collapsed. Degenerating nodule shows hypoechoic solid nodule with echogenic foci.
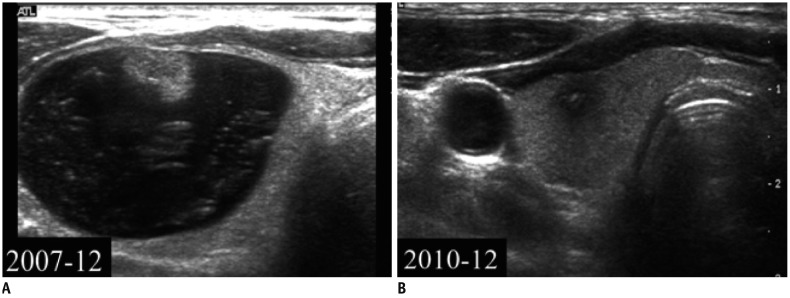
Fig. 6
Degeneration of solid thyroid nodule.
A. US image showing that initial nodule was solid with inhomogeneous echogenicity. B. FNA was performed at this point. C, D. Three years later, nodule obviously collapsed. Power Doppler imaging revealed no vascular signal within nodule.
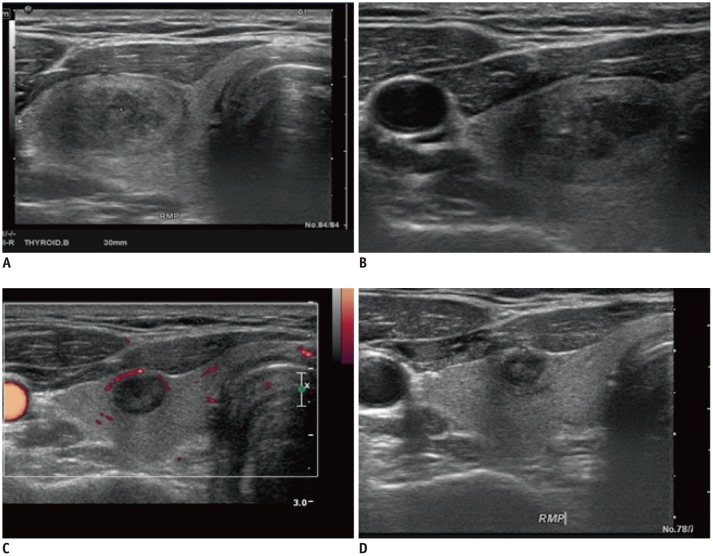
Fig. 7
Benign degenerating thyroid nodules from surgical specimen.
A. Lesion has shrunk and shows fibrotic scarring with fibrinoid degeneration (HE, × 12). B. Follicular cells are paucicellular or virtually absent (HE, × 40). C. Well-circumscribed nodule shows cystic degeneration and calcification with peripheral fibrosis (HE, × 12). D. There are no atypical cells (HE, × 40). B and D are magnified views of A and C, respectively.
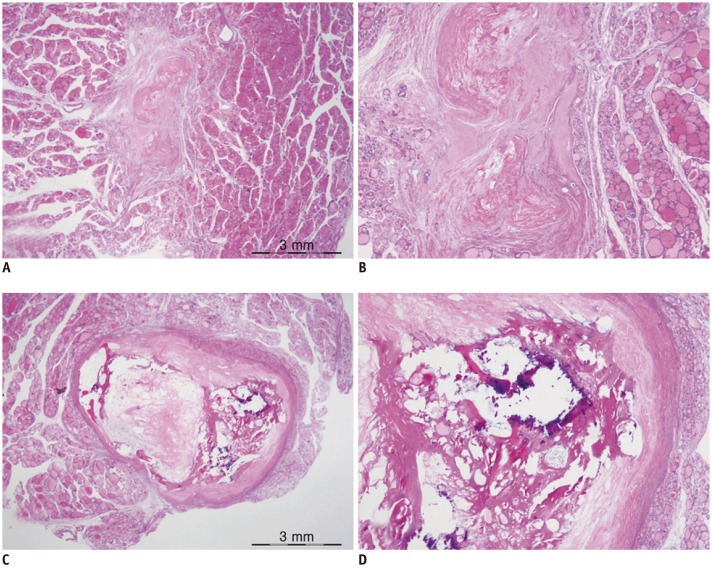
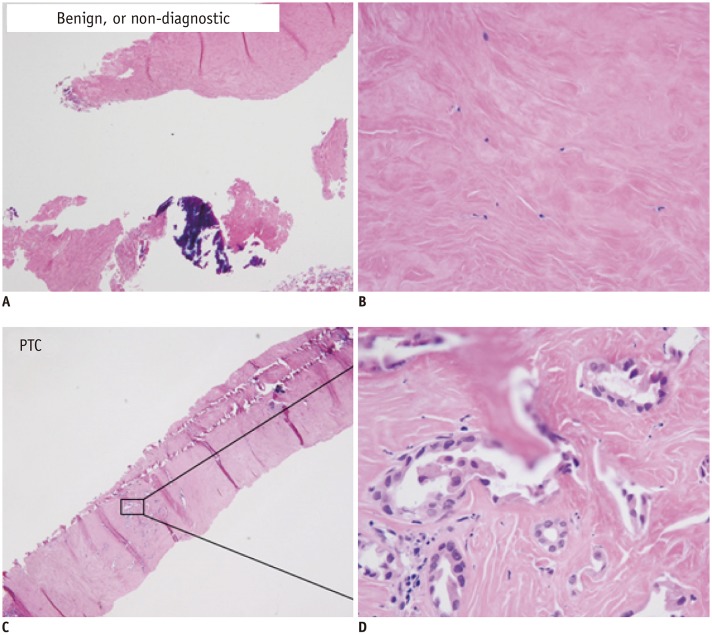
Fig. 8
CNB specimen of benign degenerating thyroid nodules (A, B) and PTC (C, D).
Benign degenerating nodule shows no atypical cells (HE, × 40, A; HE, × 400, B). Paucicellular variant of PTC shows a few atypical follicular cells with typical nuclear features of PTC (HE, × 40, C; HE, × 400, D). PTC = papillary thyroid carcinoma




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download