1. Kennedy P, Wagner M, Castéra L, Hong CW, Johnson CL, Sirlin CB, et al. Quantitative elastography methods in liver disease: current evidence and future directions. Radiology. 2018; 286:738–763. PMID:
29461949.

2. Herrmann E, de Lédinghen V, Cassinotto C, Chu WC, Leung VY, Ferraioli G, et al. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: an individual patient data-based meta-analysis. Hepatology. 2018; 67:260–272. PMID:
28370257.

3. Lupșor-Platon M, Badea R, Gersak M, Maniu A, Rusu I, Suciu A, et al. Noninvasive assessment of liver diseases using 2D shear wave elastography. J Gastrointestin Liver Dis. 2016; 25:525–532. PMID:
27981309.

4. Amur S, LaVange L, Zineh I, Buckman-Garner S, Woodcock J. Biomarker qualification: toward a multiple stakeholder framework for biomarker development, regulatory acceptance, and utilization. Clin Pharmacol Ther. 2015; 98:34–46. PMID:
25868461.

5. Li C, Zhang C, Li J, Huo H, Song D. Diagnostic accuracy of real-time shear wave elastography for staging of liver fibrosis: a meta-analysis. Med Sci Monit. 2016; 22:1349–1359. PMID:
27102449.

6. Shan QY, Liu BX, Tian WS, Wang W, Zhou LY, Wang Y, et al. Elastography of shear wave speed imaging for the evaluation of liver fibrosis: a meta-analysis. Hepatol Res. 2016; 46:1203–1213. PMID:
26857658.

7. Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, et al. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (long version). Ultraschall Med. 2017; 38:e16–e47.

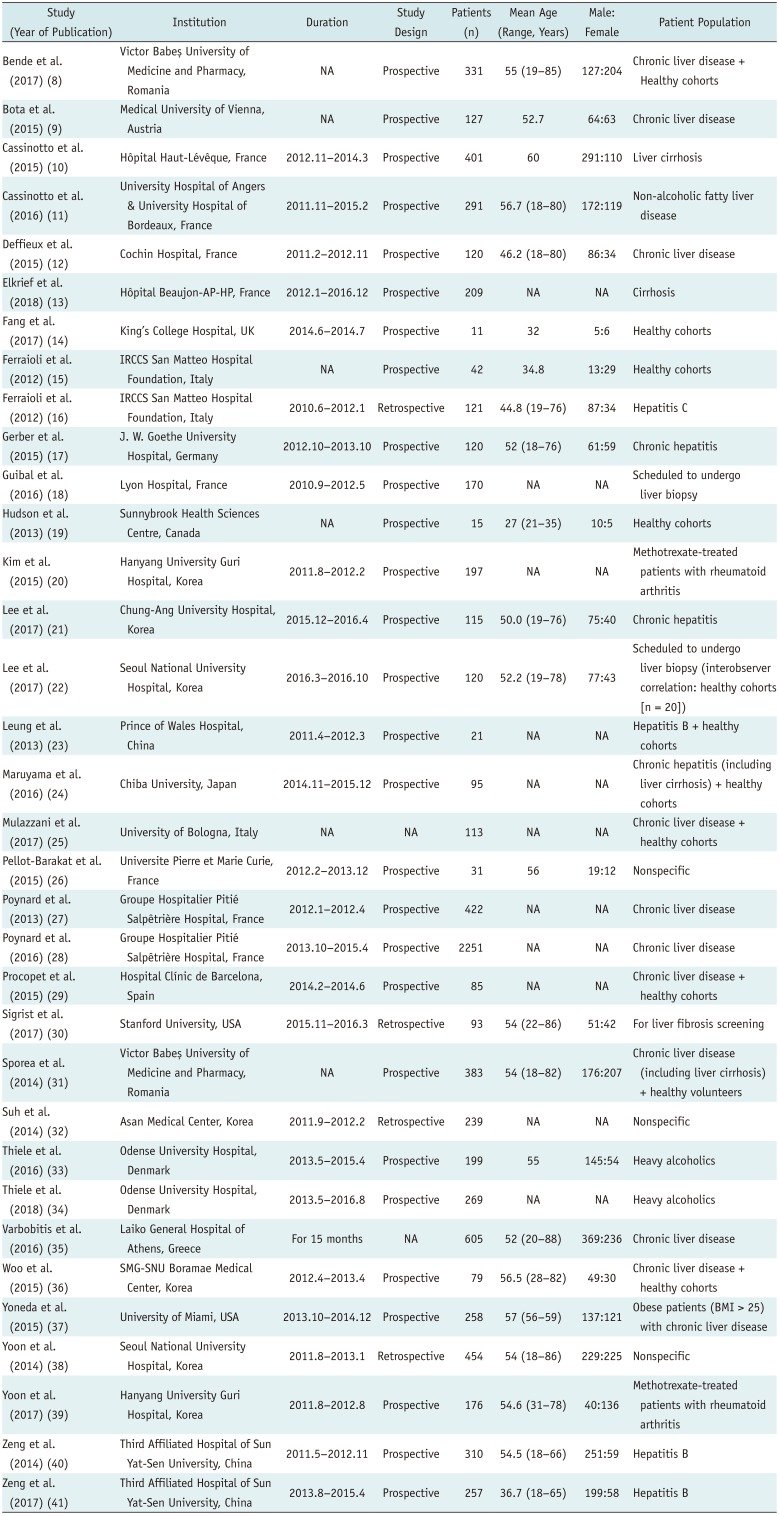
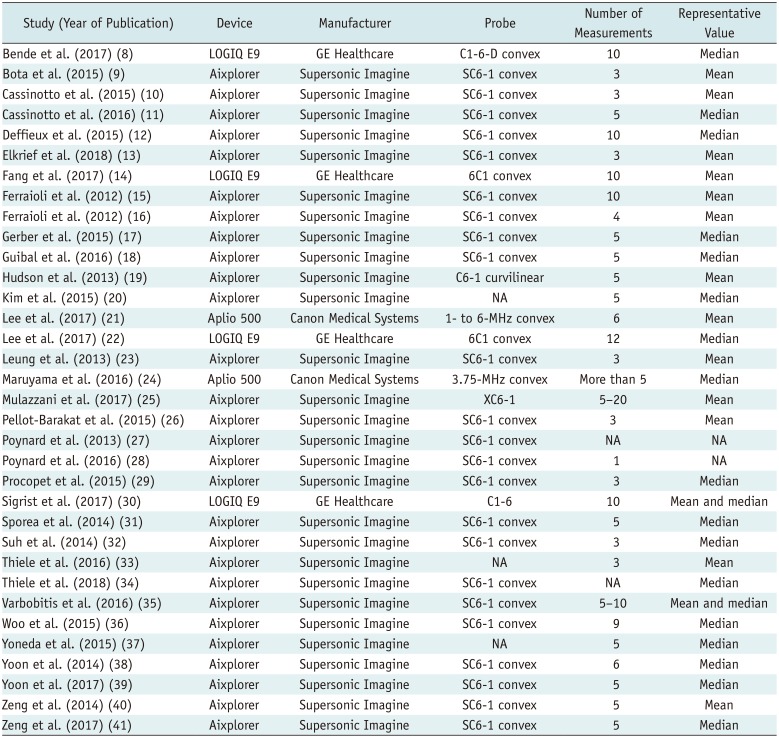
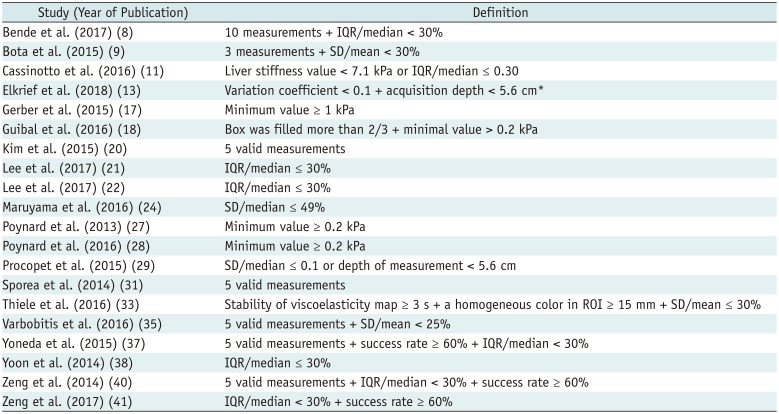
8. Bende F, Sporea I, Sirli R, Popescu A, Mare R, Miutescu B, et al. Performance of 2D-SWE.GE for predicting different stages of liver fibrosis, using transient elastography as the reference method. Med Ultrason. 2017; 19:143–149. PMID:
28440347.

9. Bota S, Paternostro R, Etschmaier A, Schwarzer R, Salzl P, Mandorfer M, et al. Performance of 2-D shear wave elastography in liver fibrosis assessment compared with serologic tests and transient elastography in clinical routine. Ultrasound Med Biol. 2015; 41:2340–2349. PMID:
26004669.

10. Cassinotto C, Charrie A, Mouries A, Lapuyade B, Hiriart JB, Vergniol J, et al. Liver and spleen elastography using supersonic shear imaging for the non-invasive diagnosis of cirrhosis severity and oesophageal varices. Dig Liver Dis. 2015; 47:695–701. PMID:
25959234.

11. Cassinotto C, Boursier J, de Lédinghen V, Lebigot J, Lapuyade B, Cales P, et al. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016; 63:1817–1827. PMID:
26659452.

12. Deffieux T, Gennisson JL, Bousquet L, Corouge M, Cosconea S, Amroun D, et al. Investigating liver stiffness and viscosity for fibrosis, steatosis and activity staging using shear wave elastography. J Hepatol. 2015; 62:317–324. PMID:
25251998.

13. Elkrief L, Ronot M, Andrade F, Dioguardi Burgio M, Issoufaly T, Zappa M, et al. Non-invasive evaluation of portal hypertension using shear-wave elastography: analysis of two algorithms combining liver and spleen stiffness in 191 patients with cirrhosis. Aliment Pharmacol Ther. 2018; 47:621–630. PMID:
29322599.

14. Fang C, Konstantatou E, Romanos O, Yusuf GT, Quinlan DJ, Sidhu PS. Reproducibility of 2-dimensional shear wave elastography assessment of the liver: a direct comparison with point shear wave elastography in healthy volunteers. J Ultrasound Med. 2017; 36:1563–1569. PMID:
28370146.

15. Ferraioli G, Tinelli C, Zicchetti M, Above E, Poma G, Di Gregorio M, et al. Reproducibility of real-time shear wave elastography in the evaluation of liver elasticity. Eur J Radiol. 2012; 81:3102–3106. PMID:
22749107.

16. Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Filice G, Filice C. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012; 56:2125–2133. PMID:
22767302.

17. Gerber L, Kasper D, Fitting D, Knop V, Vermehren A, Sprinzl K, et al. Assessment of liver fibrosis with 2-D shear wave elastography in comparison to transient elastography and acoustic radiation force impulse imaging in patients with chronic liver disease. Ultrasound Med Biol. 2015; 41:2350–2359. PMID:
26116161.

18. Guibal A, Renosi G, Rode A, Scoazec JY, Guillaud O, Chardon L, et al. Shear wave elastography: an accurate technique to stage liver fibrosis in chronic liver diseases. Diagn Interv Imaging. 2016; 97:91–99. PMID:
26655870.

19. Hudson JM, Milot L, Parry C, Williams R, Burns PN. Inter- and intra-operator reliability and repeatability of shear wave elastography in the liver: a study in healthy volunteers. Ultrasound Med Biol. 2013; 39:950–955. PMID:
23453379.

20. Kim TY, Kim JY, Sohn JH, Lee HS, Bang SY, Kim Y, et al. Assessment of substantial liver fibrosis by real-time shear wave elastography in methotrexate-treated patients with rheumatoid arthritis. J Ultrasound Med. 2015; 34:1621–1630. PMID:
26269292.

21. Lee ES, Lee JB, Park HR, Yoo J, Choi JI, Lee HW, et al. Shear wave liver elastography with a propagation map: diagnostic performance and inter-observer correlation for hepatic fibrosis in chronic hepatitis. Ultrasound Med Biol. 2017; 43:1355–1363. PMID:
28431795.

22. Lee SM, Lee JM, Kang HJ, Yang HK, Yoon JH, Chang W, et al. Liver fibrosis staging with a new 2D-shear wave elastography using comb-push technique: applicability, reproducibility, and diagnostic performance. PLoS ONE. 2017; 12:e0177264. PMID:
28510583.

23. Leung VY, Shen J, Wong VW, Abrigo J, Wong GL, Chim AM, et al. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology. 2013; 269:910–918. PMID:
23912619.

24. Maruyama H, Kobayashi K, Kiyono S, Sekimoto T, Kanda T, Yokosuka O. Two-dimensional shear wave elastography with propagation-based reliability assessment for grading hepatic fibrosis and portal hypertension. J Hepatobiliary Pancreat Sci. 2016; 23:595–602. PMID:
27440720.

25. Mulazzani L, Salvatore V, Ravaioli F, Allegretti G, Matassoni F, Granata R, et al. Point shear wave ultrasound elastography with Esaote compared to real-time 2D shear wave elastography with supersonic imagine for the quantification of liver stiffness. J Ultrasound. 2017; 20:213–225. PMID:
28900522.

26. Pellot-Barakat C, Lefort M, Chami L, Labit M, Frouin F, Lucidarme O. Automatic assessment of shear wave elastography quality and measurement reliability in the liver. Ultrasound Med Biol. 2015; 41:936–943. PMID:
25701517.

27. Poynard T, Munteanu M, Luckina E, Perazzo H, Ngo Y, Royer L, et al. Liver fibrosis evaluation using real-time shear wave elastography: applicability and diagnostic performance using methods without a gold standard. J Hepatol. 2013; 58:928–935. PMID:
23321316.

28. Poynard T, Pham T, Perazzo H, Munteanu M, Luckina E, Elaribi D, et al. Real-time shear wave versus transient elastography for predicting fibrosis: applicability, and impact of inflammation and steatosis. A non-invasive comparison. PLoS ONE. 2016; 11:e0163276. PMID:
27706177.

29. Procopet B, Berzigotti A, Abraldes JG, Turon F, Hernandez-Gea V, García-Pagán JC, et al. Real-time shear-wave elastography: applicability, reliability and accuracy for clinically significant portal hypertension. J Hepatol. 2015; 62:1068–1075. PMID:
25514554.

30. Sigrist RMS, El Kaffas A, Jeffrey RB, Rosenberg J, Willmann JK. Intra-individual comparison between 2-D shear wave elastography (GE system) and virtual touch tissue quantification (Siemens system) in grading liver fibrosis. Ultrasound Med Biol. 2017; 43:2774–2782. PMID:
28967501.

31. Sporea I, Bota S, Gradinaru-Taşcău O, Sirli R, Popescu A, Jurchiş A. Which are the cut-off values of 2D-shear wave elastography (2D-SWE) liver stiffness measurements predicting different stages of liver fibrosis, considering transient elastography (TE) as the reference method? Eur J Radiol. 2014; 83:e118–e122. PMID:
24380640.

32. Suh CH, Kim SY, Kim KW, Lim YS, Lee SJ, Lee MG, et al. Determination of normal hepatic elasticity by using real-time shear-wave elastography. Radiology. 2014; 271:895–900. PMID:
24555633.

33. Thiele M, Detlefsen S, Sevelsted Møller L, Madsen BS, Fuglsang Hansen J, Fialla AD, et al. Transient and 2-dimensional shear-wave elastography provide comparable assessment of alcoholic liver fibrosis and cirrhosis. Gastroenterology. 2016; 150:123–133. PMID:
26435270.

34. Thiele M, Madsen BS, Hansen JF, Detlefsen S, Antonsen S, Krag A. Accuracy of the enhanced liver fibrosis test vs fibroTest, elastography, and indirect markers in detection of advanced fibrosis in patients with alcoholic liver disease. Gastroenterology. 2018; 154:1369–1379. PMID:
29317276.

35. Varbobitis IC, Siakavellas SI, Koutsounas IS, Karagiannakis DS, Ioannidou P, Papageorgiou MV, et al. Reliability and applicability of two-dimensional shear-wave elastography for the evaluation of liver stiffness. Eur J Gastroenterol Hepatol. 2016; 28:1204–1209. PMID:
27340898.

36. Woo H, Lee JY, Yoon JH, Kim W, Cho B, Choi BI. Comparison of the reliability of acoustic radiation force impulse imaging and supersonic shear imaging in measurement of liver stiffness. Radiology. 2015; 277:881–886. PMID:
26147680.

37. Yoneda M, Thomas E, Sclair SN, Grant TT, Schiff ER. Supersonic shear imaging and transient elastography with the XL probe accurately detect fibrosis in overweight or obese patients with chronic liver disease. Clin Gastroenterol Hepatol. 2015; 13:1502–1509.e5. PMID:
25804329.

38. Yoon JH, Lee JM, Han JK, Choi BI. Shear wave elastography for liver stiffness measurement in clinical sonographic examinations: evaluation of intraobserver reproducibility, technical failure, and unreliable stiffness measurements. J Ultrasound Med. 2014; 33:437–447. PMID:
24567455.
39. Yoon K, Jeong WK, Kim Y, Kim MY, Kim TY, Sohn JH. 2-dimensional shear wave elastography: interobserver agreement and factors related to interobserver discrepancy. PLoS ONE. 2017; 12:e0175747. PMID:
28414822.

40. Zeng J, Liu GJ, Huang ZP, Zheng J, Wu T, Zheng RQ, et al. Diagnostic accuracy of two-dimensional shear wave elastography for the non-invasive staging of hepatic fibrosis in chronic hepatitis B: a cohort study with internal validation. Eur Radiol. 2014; 24:2572–2581. PMID:
25027837.

41. Zeng J, Zheng J, Huang Z, Chen S, Liu J, Wu T, et al. Comparison of 2-D shear wave elastography and transient elastography for assessing liver fibrosis in chronic hepatitis B. Ultrasound Med Biol. 2017; 43:1563–1570. PMID:
28483579.

42. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009; 151:W65–W94. PMID:
19622512.

43. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155:529–536. PMID:
22007046.

44. Suh CH, Park SH. Successful publication of systematic review and meta-analysis of studies evaluating diagnostic test accuracy. Korean J Radiol. 2016; 17:5–6. PMID:
26798211.

45. Kim KW, Lee J, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part I. General guidance and tips. Korean J Radiol. 2015; 16:1175–1187. PMID:
26576106.

46. Lee J, Kim KW, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part II. Statistical methods of meta-analysis. Korean J Radiol. 2015; 16:1188–1196. PMID:
26576107.

47. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003; 327:557–560. PMID:
12958120.

48. Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration Web site. Updated March 2011. Accessed January 8, 2017.
https://handbook-5-1.cochrane.org/.
49. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634. PMID:
9310563.

50. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000; 56:455–463. PMID:
10877304.

51. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010; 36:1–48.
52. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016; 15:155–163. PMID:
27330520.

53. Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010; 51:828–835. PMID:
20063276.

54. Wang CZ, Zheng J, Huang ZP, Xiao Y, Song D, Zeng J, et al. Influence of measurement depth on the stiffness assessment of healthy liver with real-time shear wave elastography. Ultrasound Med Biol. 2014; 40:461–469. PMID:
24361224.
55. Barr RG, Ferraioli G, Palmeri ML, Goodman ZD, Garcia-Tsao G, Rubin J, et al. Elastography assessment of liver fibrosis: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2015; 276:845–861. PMID:
26079489.

56. Buckler AJ, Bresolin L, Dunnick NR, Sullivan DC, Aerts HJ, Bendriem B, et al. Quantitative imaging test approval and biomarker qualification: interrelated but distinct activities. Radiology. 2011; 259:875–884. PMID:
21325035.

57. Sporea I, Bota S, Jurchis A, Sirli R, Grădinaru-Tascău O, Popescu A, et al. Acoustic radiation force impulse and supersonic shear imaging versus transient elastography for liver fibrosis assessment. Ultrasound Med Biol. 2013; 39:1933–1941. PMID:
23932281.

58. Sporea I, Grădinaru-Taşcău O, Bota S, Popescu A, Şirli R, Jurchiş A, et al. How many measurements are needed for liver stiffness assessment by 2D-shear wave elastography (2D-SWE) and which value should be used: the mean or median? Med Ultrason. 2013; 15:268–272. PMID:
24286089.

59. Singh S, Loomba R. Role of two-dimensional shear wave elastography in the assessment of chronic liver diseases. Hepatology. 2018; 67:13–15. PMID:
28777887.

60. Ferraioli G, Filice C, Castera L, Choi BI, Sporea I, Wilson SR, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 3: liver. Ultrasound Med Biol. 2015; 41:1161–1179. PMID:
25800942.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download