Since 1997, A/goose/Guangdong/1/1996 (Gs/GD) H5N1 lineage of highly pathogenic avian influenza (HPAI) viruses have circulated in many poultry farm and wild birds. It has caused severe economic damage in many counties [
1]. The H5N1 Gs/GD lineage HPAI virus has evolved into distinct genetic clades. Its pathogenicity also varies in different hosts [
2].
In Korea, 7 HPAI outbreaks have emerged since 2003. There were 4 outbreaks of H5N1 virus from 2003 to 2011, H5N8 virus in 2014, H5N6 and H5N8 virus in 2016 and 2017, and H5N6 in 2017 and 2018 [
345678]. During the winter of 2016 and 2017, H5N6 and H5N8 HPAI viruses emerged in a series. These outbreaks caused the highest economic damage and the largest culling, of about 38 million poultries. Notably, the H5N6 outbreak caused about 27 million chickens culling. Out of these, more than 23 million chickens were brown line chickens (layer chickens, layer parent stock, and Korean native chickens [KNCs]) and about 4 million were white line chickens (broilers and broiler parent stock) [
9]. In this outbreak, the H5N6 viruses that were isolated from wild birds and poultry farms in South Korea were divided into 5 genetic groups [
7]. A/duck/Korea/ES2/2016 (ES2/16 H5N6) H5N6 representative HPAI virus within the clade 2.3.4.4 belonged to the most dominant group. Therefore, we compared the pathogenicity and transmissibility in the specific pathogen-free (SPF) chickens of ES2/16 (H5N6) virus with those of the HPAI virus that occurred in Asia. We also compared the pathogenicity in different chicken lines and the KNCs of brown chicken lines with broilers of white chicken lines.
The ES2/16 (H5N6) virus was propagated in the allantoic cavities of 9- to 11-day-old SPF embryonated chicken eggs.
Six-week-old SPF chickens (white Leghorn), 3-week-old broilers (white line) and 8-week-old KNC (brown line) were used for this study. Prior to the performance of the experimental infections, blood samples were tested to certify for non-exposure to avian influenza (AI) virus by hemagglutination inhibition tests using the World Animal Health Organisation (OIE) manual. Each breed was housed separately in a negative pressure and high efficiency air-filtered isolator. All experiments were performed in biosafety level 3 containment facilities. All the animal experiments were approved by the Animal Ethics Committee of the Animal and Plant Quarantine Agency of Korea (approval No. 2016–345).
To assess the pathogenicity, intravenous pathogenicity tests with 6-week-old SPF chickens were performed according to the instructions in the OIE manual. Ten 6-week-old SPF chickens were inoculated via the intravenous route with a 0.2 mL of 1:10 dilution of a bacteria-free allantoic fluid, containing 107.7 50% egg infective doses (EID50) of the ES2/16 (H5N6) virus.
To evaluate the chicken lethal dose 50 (cLD50), each breed of chicken was divided into 4 groups (n = 5) and intranasally inoculated serial 10-fold dilutions, ranging from 103 to 107 EID50 titers of ES2/16 (H5N6) virus (SPF chickens, broilers: 103–106, KNC: 104–107 EID50).
To compare pathogenicity and transmission depending on the chicken breeds, 8 chickens of each breed were intranasally inoculated with 0.1 mL 106 EID50 of ES2/16 (H5N6) virus. Ten hours later, 3 contact chickens were co-housed with the infected chicken breed, respectively. Two chickens per breed were intranasally inoculated with 0.1 mL of phosphate buffered saline as control.
Oropharyngeal (OP) and cloacal (CL) swabs were collected for 1, 2, 3, 4, 5, 6, 7, 10, and 14 days post-infection (dpi) to evaluate the viral shedding. At 3 dpi, 3 chickens of each inoculated group were sacrificed and necropsied to collect eleven organs, which were used for tissue tropism and histopathological analysis. The chicken embryo fibroblast cells were used to determine the median tissue culture infective dose (TCID50). The virus titer values were calculated using the method of Reed and Muench (1938).
All the SPF chickens inoculated with ES2/16 (H5N6) virus intravenously with 0.2 mL of 1:10 dilution were dead within 1 day. The intravenous pathogenicity index of the ES2/16 (H5N6) virus in chickens was found to be 3.0. This was by classifying the viruses as HPAI according to the OIE manual.
All the SPF chickens inoculated with 10
6.0 EID
50/0.1 mL of ES2/16 (H5N6) virus showed 100% mortality and their mean death time (MDT) was 2.6 days (
Table 1). A previous study showed that MDT in SPF chickens of A/Chicken/Korea/ES/03 (H5N1, ES/03) and A/Chicken/Korea/IS/06 (H5N1, IS/06) were 2.0 and 3.0 days, respectively [
4]. The A/broiler duck/Korea/Buan2/14 (H5N8, Buan2/14) were 4.5 days [
4610]. The MDT of ES2/16 (H5N6) virus was similar to those of the ES/03 (H5N1) and IS/06 (H5N1) viruses. However, they were shorter than those of the Buan2/14 (H5N8) virus.
The cLD
50 value of the ES2/16 (H5N6) virus was 10
3.7 EID
50 in the SPF chickens (
Table 1). The cLD
50 of ES2/16 (H5N6) was similar to those of many H5N1 HPAI viruses (10
2.5–10
3.5 EID
50), which had emerged in Asia or Korea from 2003 to 2008. However, it was much lower than that of the Buan2/14 (H5N8) (10
5.3 EID
50) [
6] and A/chicken/Kumamoto/17/2014 (H5N8) viruses (10
5.8 EID
50) [
11]. Notably, the cLD
50 for the ES2/16 (H5N6) virus was lower than that for the A/black swan/Akita/1/2016 (H5N6, Akita/16) virus (10
4.3 EID
50), although both ES2/16 (H5N6) and Akita/16 (H5N6) had emerged in same year and belonged to group C of the clade 2.3.4.4 [
11].
In the transmissibility experiment, the ES2/16 (H5N6) virus caused a 100% mortality rate of the contact group in the SPF chickens. In previous studies, the contact group of IS/06 (H5N1) virus showed 100% mortality rate, whereas those of Buan2 (H5N8) and A/breeder duck/Korea/Gochang1/2014 (H5N8) viruses showed 66.6% and 33.3% mortality rate, respectively [
810]. This data suggests that the ES2/16 (H5N6) virus can be transmitted between chickens, which behaved similarly to the H5N1 virus in SPF chickens.
Furthermore, we compared the pathogenicity of the ES2/16 (H5N6) virus in different chicken lines, broilers of white chicken line and KNC of brown chicken line. The MDT for the ES2/16 (H5N6) were slightly longer in the KNC (3.1 days) than those in the broilers (2.1 days) (
Table 1). The cLD
50 of the ES2/16 (H5N6) virus in the KNC (10
4.3 EID
50) were also slightly higher than those in the broilers (10
3.8 EID
50) (
Table 1). In the transmissibility experiment, the ES2/16 (H5N6) virus caused 100% mortalities to both the KNC and broilers contact chickens. The MDT of contact KNC was 6.9 days, which was also longer than that of the contact broiler, which was 3.9 days. The 2 white lines of broilers and SPF chickens showed a similar MDT and cLD
50 in the direct infection and transmissibility experiment. The KNC seemed to be more resistant to the ES2/16 (H5N6) virus than broilers. This result was consistent with a previous study that brown lines have more resistance to the AI [
61213]. The brown breed is thought to be relatively resistant to HPAI. Accordingly, there is high possibility that KNC is resistant to HPAI, considering it belongs to the same breed. Interestingly, the pathogenicity of the ES2/16 (H5N6) virus between the white and brown chicken lines had less variation than that of the Buan2/14 (H5N8) virus [
6].
OP and CL samples were taken on a daily basis from the all intranasally inoculated and contact chickens. In the inoculated SPF chickens and broilers, the ES2/16 (H5N6) virus was detected from 1 dpi until 3 or 4 dpi in the OP and CL swabs. However, in those of the KNC, the virus was detected from 2 dpi until 5 dpi (
Fig. 1). In the contact KNC, the ES2/16 (H5N6) virus was also shed through the OP or CL route longer period, which was from 3 to 8 dpi than in the SPF chickens or broilers, which had a period of 2 to 4 dpi (
Fig. 1). One of three chickens in the KNC contact group excreted the virus first 7 dpi and it survived until 8 dpi, which was probably caused by a secondary infection.
During the HPAI virus outbreak of the winter of 2016 and 2017 in Korea, 178 cases of H5N6 HPAI virus occurred in chicken farms. The occurrences of the brown chicken farms (layer chicken, layer parent stock, and KNC) accounted for 158 cases (88.8%). Those of the white chicken farms (broilers) accounted for 20 cases (11.2%) of the total cases. The estimated reason for the high incidence of the H5N6 virus in brown chicken farms is that all-in-all-out was systematized in the white chicken farm and the access frequency to the white chicken farms was low. Moreover, the frequency of egg carrier vehicles in the layer farms was high and contributed to the farm-to-farm transmission. The characteristic that the KNC of the brown line have a longer virus shedding period and MDT might also have contributed to the distribution of farm-to-farm transmission in the 16/17 H5N6 HPAI virus outbreak.
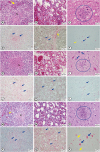
All the breed chickens showed pathological lesions, such as edema or necrosis, in various parenchymal tissues. Histopathological lesions were predominantly observed in the lung, spleen, liver, pancreas, kidney and brain. Particularly, the ES2/16 (H5N6) virus caused moderate to severe congestion, edema or necrosis in spleen, lung and brain in all the 3 chicken breeds (
Fig. 2). The AI virus antigens were detected in the blood vessel walls or necrotic parenchymal cells of all the tested organs, such as the trachea, lung, spleen, liver, pancreas, muscle, cecal tonsil, intestine, kidney, brain, and heart, in immunohistochemical analysis (
Fig. 2). Therefore, we confirmed that the ES2/16 (H5N6) virus circulated the entire body of a chicken, regardless of the chicken breed.
In the viral distribution experiment, the ES2/16 (H5N6) virus was isolated in all the tested tissues of the 3 chicken breeds with a viral titer higher than 5.2 log
10TCID
50/0.1 mL. This means that the ES2/16 (H5N6) virus caused a systemic infection in the 3 chicken breeds (
Supplementary Fig. 1).
We evaluated the pathogenicity of the ES2/16 (H5N6) HPAI virus, which caused the highest economic damage and largest culling to the poultry farms in the HPAI outbreaks in Korea. The ES2/16 (H5N6) virus could infect chickens with small doses leading to rapid death. However, the KNC of the brown chicken line shed the ES2/16 (H5N6) virus longer than the broilers of the white chicken lines. This characteristic of the brown chicken line in the H5N6 HPAI virus could be one of the reasons that many brown chicken farms were affected in the H5N6 HPAI virus. Therefore, a pathogenicity test is needed for any new emerging H5 HPAI virus.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download