Background
Fluoropolymers are fluorinated carbon-based polymers with multiple carbon-fluorinated bonds [
1]. Fluoropolymers have properties of lubricity, chemical inertness, strength, plasticity, and thermal stability. These materials are widely used in gaskets, coating, self-lubricating bearings, food manufacturing machinery, household products such as nonstick cooking utensils, and other applications [
2].
Acute lung toxicity from PTFE fumes and chronic foreign body reactions from injected PTFE have been reported. PTFE degrades at temperatures higher than 360 °C, produces toxic fumes, and causes severe lung injury [
3,
4]. Fluorocarbon-containing aerosol product exposure due to spraying can also cause acute lung injury [
5–
7]. Chronic lung disease found in PTFE-spraying workers has been reported [
8]. However, the pathophysiology of PTFE particle-induced chronic lung disease has not been reported. Furthermore, measurement of the airborne concentration of PTFE particles has not been reported.
Here, using analysis of air samples from a workplace, we report one case of small airway-centered granulomatosis pneumonitis after long-term exposure to the PTFE spray-coating process. An exposure assessment was also performed. The present study protocol was reviewed and approved by the institutional review board of Keimyung University Dongsan Medical Center (IRB No. 2016–02–024-005).
Conclusion
This is a case report of small airway-centered granulomatosis caused by PTFE particles from the spraying process with a description of the air concentration of PTFE in a workplace. The patient was diagnosed with granulomatous lung disease from PTFE using CT and lung pathology and electron microscopic findings, which are compatible with a previously reported case [
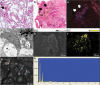
8]. We confirmed the presence of PTFE particles in the lung tissue by SEM and EDX of lung tissue. Additionally, the air sample from the workplace was analyzed by FT-IR, EDX, and TGA. The presence of PTFE was confirmed, and the diameter of the particles was measured. The air concentration was also calculated.
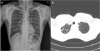
The patient's CT findings showed numerous tiny scattered nodules and a few calcified nodules in both lungs; however, these were distinguishable from those of classic silicosis. The CT findings of typical silicosis include upper-lobe-dominant peri-lymphatic distribution of multiple 2–5 mm nodules with hilar and mediastinal lymph node enlargement and calcification [
10]. Therefore, we ruled out silicosis as a diagnosis in this patient.
The respiratory effects of PTFE are usually focused on acute toxicity. Heated PTFE particles may cause symptoms that range from mild flu-like symptoms to severe symptoms, such as pulmonary edema [
11,
12]. Various previous studies have suggested that ultrafine particles from the heating of PTFE severely injure the lungs, and the particles lose their toxicity after becoming coagulated into larger homogeneous particles [
4,
13]. Acute pulmonary toxicity due to fluorocarbon-containing aerosol spray has been reported [
14] from various work processes, such as those of waterproof leather, fabric spray, floor-stain protector, rust-proofing spray, grout sealer, and ski wax [
15–
18]. Choi et al. reported for the first time chronic pulmonary granulomatosis associated with exposure to PTFE [
8]. The spraying process and aerosolized PTFE were excluded as the cause of small airway-centered granulomatosis because of the stability of PTFE in a liquid formulation [
8]. However, nondegraded PTFE can induce an immunologic reaction in body tissue. PTFE has been used in various medical processes because it is well tolerated by the body tissue, not resorbed, and disperses in various fluids. However, foreign-body granulomatous reactions after the injection of PTFE have been reported, including Teflon granuloma formation after microvascular decompression [
19], vocal cord injection for treating paralyzed vocal cords [
20], suburethral injection for the treatment of vesicoureteral reflux in children [
21], acetabular cup for hip replacement [
22], and as a bulking agent for the treatment of stress urinary incontinence [
23] has been reported. Foreign-body giant cell reaction and a glassy-appearing material in multinucleated giant cells are typical pathological findings of Teflon-induced foreign-body reaction [
24]. Like these cases, multinucleated giant cells containing glassy-appearing material were frequently noted in the present case.
This patient worked on the same process for 28 years and never worked on other processes, including the heat-drying process. In our study, we collected both personal and regional samples from the workplace and analyzed them to identify the cause of small airway-centered granulomatosis. We confirmed the presence of PTFE particles of up to 20 μm by FT-IR, EDX, and TGA from the personal air samples. FT-IR, SEM and EDX analysis of the patient's lung tissue showed the presence of 2–6 μm PTFE particles. The size of the pyrolyzed PTFE particles was 0.02–0.2 μm at 560 °C and 0.02–0.07 μm at 370 °C. Although pyrolyzed PTFE can aggregate into larger particle size, only particles pyrolyzed from high temperature up to 560 °C aggregated into large globular agglomerates, while particles pyrolyzed from 370 °C aggregated into chain shapes up to 1.6 μm in size [
25]. In our study, the PTFE particles from air sampling measured 1–22 μm by electron microscopy, and the size corresponded to reported PTFE powder size (7.6 ± 8.5 μm) [
26]. The particle size identified in the lungs was 2–6 μm. The coating process occurred at 180–400 °C; the size of pyrolyzed particles formed at this temperature would be smaller. Additionally, aggregated pyrolytic products of PTFE showed variety in shape such as spherical, undulating, concave, bowl or doughnut-shaped with a thickened peripheral portion [
27]. In this study, the samples of PTFE collected from workplace revealed a round regular shape. Therefore, the particles are more likely to have originated from the spray process. As a result, we suggest that the small airway-centered granulomatosis diagnosed in this patient was caused by the aerosolized PTFE particles from the spraying process.
Patient lesions seemed to be caused by prolonged exposure to the aerosolized PTFE particles from the spraying process, without acute respiratory symptoms. One limitation of this study is that TGA was not performed on the collected lung tissue. Furthermore, the health effects of particles formed by pyrolysis cannot be excluded. When pyrolysis occurs, PTFE is decomposed into C
2F
4, C
3F
6, and C
4F
8 compounds [
27]. We have not clearly excluded the presence of pyrolyzed PTFE particles such as CF
2 = CF
2, CF
3 - CF = CF
2 other than C-F bonds. In further studies, it will be necessary to quantitatively confirm pyrolyzed and non-pyrolyzed particles by separating samples according to particle size using an impactor and analyzing the samples via GC-MS. Epidemiological studies of chronic lung disease in workers using PTFE spray will also be needed.
In Korea, work environment monitoring is performed for metal dust, mineral dust containing silica, and several other dusts that are regulated by law. However, there is no regulation for measuring or controlling the concentration of many respirable particles, such as PTFE. Furthermore, no time-weighted average or short-term exposure limit is suggested for PTFE. Acute and chronic pulmonary diseases caused by PTFE have been reported, and further studies should be conducted to recognize toxicity and establish an exposure limit for PTFE. This study, with its quantitative analysis of the airborne concentration of PTFE, suggests a hazardous airborne concentration of PTFE and may support setting an exposure limit for PTFE.





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download