Background
IgA nephropathy (IgAN) is a glomerular disease characterized by IgA deposition in the glomerular mesangium of the kidney, which was first reported by Berger et al. in 1968 [
1]. IgAN is the most common form of glomerulonephritis in the world, accounting for about 30–45 % of all primary glomerular diseases [
2]. IgAN can occur in all age groups, but is mainly found in patients in their 10s–30s, and it is more common in males, with a male to female gender ratio of 2:1 [
3,
4]. The disease can be diagnosed through biopsy by visualization of IgA deposition in the glomerular mesangial area using immunofluorescence microscopy [
2,
4]. IgAN presents with macroscopic hematuria in about 40 to 45 % of patients, with microscopic hematuria and proteinuria in about 35 to 40 %, and with nephrotic syndrome or acute renal failure in the remainder [
3,
5]. While IgAN has an indolent course, about 30 % of patients will reach end-stage renal disease (ESRD) after 20 years, particularly in those who present with hypertension, heavy proteinuria or renal insufficiency [
3–
5]. Still no treatment is known to modify mesangial deposition of IgA, and available treatment options are directed to mostly at downstream immune and inflammatory events that may lead on to renal scarring [
4]. Although the etiology and pathophysiology of IgAN have not yet been clearly identified, accumulating evidence suggests that the onset and progression are caused by interactions between various genetic and environmental factors [
6–
9]. Among them, occupational risk factors such as exposure to organic solvents have been suggested as probable causes that may induce [
7,
8,
10] or aggravate IgAN [
11–
13].
The present case report is the first report in Korea suggesting the possibility that occupational exposure to organic solvents such as toluene and dichloromethane, commonly used in laboratories and industrial fields, can act as a risk factor for the onset and/or progression of IgAN. The present case report attempted to investigate the association of occupational solvent exposure and IgAN based on patient clinical information, inspection of the working environment, exposure assessment, and review of related literature, by which the authors aimed to contribute to the approach and prevention of other cases that may occur in the future.
Discussion
A number of studies have reported kidney toxicity of organic solvents and an association with glomerulonephritis. Miller et al. [
15] reported a case of acute tubular necrosis accompanied by acute renal failure, myoglobinuria, and liver enzyme elevations that were caused by inhalation exposure to dichloromethane. They conclude that dichloromethane may have potential as both a hepatotoxic and nephrotoxic agent when inhaled at high concentrations over an extended period of time [
15]. Ana-Lilia et al. [
16] reported that shoe-workers who were occupationally exposed to toluene-based glues had a higher risk of renal glomerular and/or tubular damage than the control group. Ravnskov et al. [
17] conducted a meta-analysis of 14 case–control studies and reported that the combined data support the hypothesis that hydrocarbon exposure increases the risk of chronic renal disease, although no specific hydrocarbons were identified in these reviews. In addition, there have also been other case reports [
18], epidemiological studies [
8,
19,
20] and animal-experimental studies [
19,
21] reporting associations between solvent exposure and onset of glomerulonephritis or deterioration of renal function. On the other hand, a few studies [
22,
23] reported negative results on the association between solvent exposure and the risk of chronic glomerulonephritis.
In the clinical literature that specifically reported IgAN in relation to occupational exposure, several case studies reported the onset of IgAN due to exposure to organic solvents [
24,
25], in addition to cadmium [
24,
26,
27] and silica [
28] (Table
4). Albrecht et al. [
25] reported the case of a 28-year-old male pipe plumbing worker who developed IgAN after occupational exposure to organic solvents. The corresponding patient was exposed to organic solvents during 9 years and 6 months of pipe plumbing work, resulting in the symptom of gross hematuria, and he was diagnosed with IgAN by a biopsy [
25]. Working environment measurement identified that the patient mainly carried out pipe plumbing tasks in closed bathrooms, during which he was exposed to a high concentrations (389–757 ppm) of tetrahydrofuran (THF), a component of polyvinyl chloride (PVC) pipe cement (the short-term exposure limit for THF set by the NIOSH is 250 ppm) [
25]. The authors stated that a predisposition toward IgAN in this patient could be exacerbated by massive short-term exposure to the solvent [
25].
Table 4
Case reports of IgAN associated with occupational solvents exposure

|
Author(s) (year) |
Gender/Age at diagnosis |
Occupation |
Type of solvent |
Exposure duration |
Biopsy confirm |
Prognosis |
|
Albrecht et al. [25] (1987) |
Male/28y |
A plumber, working with solvent-based pipe cement |
tetrahydrofuran (THF) |
9 years and 6 months |
+ |
not identified |
|
Fernandez et al. [24] (2010) |
Male/47y |
A pesticide manufacturer |
various organic solvents (toluene, xylene, acetone, cyclohexanone, etc.) |
23 years |
+ |
progression to CRF in 2 years after diagnosis |
Meanwhile, Fernandez et al. [
24] introduced a case of IgAN in a 50-year-old male who was exposed to various types of organic solvents for 23 years. The male was continuously exposed to organic solvents occupationally for an additional two years even after diagnosis with IgAN, resulting in progression to CRF stage 3 in two years after diagnosis with IgAN [
24].
Literature searches on epidemiologic studies that analyzed correlations between IgAN and occupational solvent exposure resulted in a total of three case–control studies reported to date [
10,
11,
29] (Table
5). First, Porro et al. [
10] investigated occupational and non-occupational exposures to organic solvents in 60 chronic glomerulonephritis patients diagnosed by biopsy in the Bari area of Southern Italy and a control group (60 nephrolithiasis and 60 traumatic fracture patients) from 1983 to 1987. The odds ratio of chronic glomerulonephritis for patients occupationally exposed to solvents was 3.9 (95 % confidence interval [CI] 1.64–8.33), and a logistic regression model showed a dose–response relationship of occupational exposures to solvents and glomerulonephritis [
10]. When IgAN patients (
n = 27) were separately evaluated, an increased risk was found for both total (Relative risk [RR] = 3.5, 95 % CI 1.18–12.18) and occupational exposure (RR = 4.25, 95 % CI 1.18–16.36) [
10].
Table 5
Epidemiological studies investigating the association of occupational solvent exposure and IgAN

|
Author(s) (year) |
Study design |
Subjects |
Outcome |
Period |
Population Total GNa/IgAN |
Resultse
|
|
Porro et al. [10] (1992) |
case–control study |
60 biopsy-proven chronic GNa patients in a University hospital in Italy (including 27 IgAN patients) |
onset of IgAN |
1987–1990 |
60/27 (reference:120) |
·An increased risk of IgAN was found for occupational solvent exposure group.
·RRb of IgAN for occupational solvent exposure ; 4.25 (1.18–16.36) |
|
Stengel et al. [11] (1995) |
case–control study |
298 biopsy-proven GNa patients in 5 hospitals in Paris (including 116 IgAN patients) |
aggravation of IgAN to CRF |
1989–1991 |
298/116 (reference:298) |
·Among males, clear association was observed between CRF and high exposure to solvents for IgAN; ORc = 3.5 (1.0–11.8), P < 0.05.
·The OR increased with duration of exposure; OR = 5.6 (1.3–24.1) for ≥10 years exposure, (P = 0.02).
·No relationship was observed for cases without CRF. |
|
Wakai et al. [29] (1999) |
case–control study |
94 biopsy-proven IgAN patients in medical centers in Japan |
onset of IgAN |
1997–1999 |
94/94 (reference:185) |
· Work-related exposure to organic solvents was found not to be associated with the risk for IgAN; OR = 0.55 (0.27–1.12), (P < 0.10) |
|
Jacob et al. [12] (2007) |
retrospective cohort study |
338 non-ESRD patients in Paris (including 194 IgAN patients with biopsy confirm) |
aggravation of IgAN to ESRD |
2002–2004 |
338/194 |
·Solvent exposure was associated with faster progression of IgAN to ESRD, HRd for IgAN is 2.6 (1.3–5.5) for high exposure versus none (p < 0.05).
·There was a trend increasing HR with exposure duration before and its persistence after diagnosis. |
|
Jacob et al. [13] (2007) |
retrospective cohort study |
269 patients with non-ESRD and biopsy-proven primary GNa diagnosis between 1994 and 2001 in Paris and suburbs (including 167 IgAN patients) |
aggravation of IgAN to ESRD |
2002–2004 |
269/167 |
·This study showed the potential role of toluene and xylene, some petroleum products, ketones and possibly dichloromethane in the progression of GNa to ESRD. |
In contrast, a case–control study that was conducted by Wakai et al. [
29] with Japanese subjects to investigate a correlation between diagnosis of IgAN and occupational exposure to organic solvents showed an odds ratio of 0.55 (95 % CI 0.27–1.12), which was not statistically significant [
29]. The authors commented that they might have failed to find a positive association because of the few number of patients with advanced renal failure, and also pointed out that another possible limitation was the methodological difficulty of measuring exposure to organic solvent [
29].
Meanwhile, Stengel et al. [
11] performed a case–control study to investigate a correlation between organic solvent exposure and CRF with 116 IgAN patients in five hospitals in Paris. As a result, among males, a clear association was observed between CRF and high exposure to organic solvents for IgAN patients (OR = 3.5, 95 % CI 1.0–11.8) [
11]. In addition, the odds ratio increased with duration of exposure [
11]. On the other hand, patients with IgAN who had normal renal function showed no correlation with organic solvents, so these results were interpreted to suggest that organic solvents contributed to deterioration of renal function to some degree rather than to the diagnosis itself of IgAN [
11].
Meanwhile, in 2007, Jacob et al. [
12] conducted the GN-PROGRESS cohort study; the authors investigated occupational risk factors for the progression of glomerulonephritis to ESRD. Using a cohort study design, they showed that solvent exposure was indeed associated with faster progression to ESRD with IgAN (Hazard ratio [HR] = 2.6, 95 % CI 1.3–5.5) [
12]. In patients with IgAN, there was a trend in increased hazard ratios with exposure duration before and its persistence after diagnosis [
12].
In a subsequent study, using data from the GN-PROGRESS cohort, Jacob et al. [
13] therefore systematically investigated the risk of progression to ESRD, by solvent-exposed job category, type of solvent-containing products, and by solvent or solvent family. In this analysis [
13], they focused on the 269 patients with either IgAN or membranous nephropathy. Among solvents, the highest risks were found for: toluene/xylene (HR = 5.1, 95 CI 1.8–14.8), gasoline, fuel and gas-oil (HR = 8.6, 95 CI 2.7–27.4), and ketones (HR = 13.3, 95 % CI 1.4–123.5). Most interestingly, they also observed an excess risk for any exposure level to methylene chloride, also known as dichloromethane (HR = 6.4, 95 % CI 1.7–24.8) [
13]. In another occupational cohort study in US aircraft workers, Radican et al. [
30] pointed to the risk of all-cause ESRD associated with trichloroethylene, 1,1,1-trichlorethane, dichloromethane, and JP4 gasoline.
In summary, regarding the relationship between occupational solvent exposure and IgAN in the literature, all reviewed studies [
11,
12] consistently reported significant correlations at least between solvent exposure and the progression of preexisting IgAN to CRF or to ESRD, though study results regarding the onset of IgAN [
10,
11,
29] were inconsistent (Table
5). In particular, recent cohort study results [
12,
13,
30], showing the potential role of toluene and dichloromethane in the progression of IgAN to ESRD supports our hypothesis that the IgAN of this case report may have progressed more rapidly to ESRD due to occupational solvent exposure.
Although it can occur at any age, IgAN mainly occurs in young adults (16–35 years old) [
3,
31–
33]. In a Chinese cohort study of 1,115 patients with IgAN [
34], the mean age at time of initial clinical manifestations and renal biopsy were 31 ± 9, and 33 ± 9 years old, respectively. For the patient in the present case, gross hematuria was first experienced at 41 years of age in June 2000, and the serum Cr level at that time was 1.3 mg/dL, corresponding to a normal level. In July 2001, he was given a clinical diagnosis of IgAN (not biopsy confirmed), which was when the patient was 42 years old. This is much older than the usual age for initial clinical presentation and diagnosis of IgAN compared to the mean age of diagnosis (10–30 years) reported in other studies [
31,
32,
34], so it was suspected that this case could be different from the general cases that were diagnosed at earlier ages, at about 10–30 years, mostly due to internal or genetic factors.
For the natural history of IgAN, the actuarial renal survival rate for 10 years after diagnosis or after a biopsy is a significant parameter for prognosis prediction of IgAN [
31,
35]. According to a meta-analysis by Coppo [
35], the actuarial renal survival rate for 10 years after diagnosis with IgAN or after a biopsy was mostly about 80–90 % in Europe, Asia and Australia. Another study conducted in United Kingdom, using Medical Research Council’s Glomerulonephritis Registry [
36], reported that the 10-year cumulative renal survival rate was 83.3 %. Le et al. [
34] reported a cohort study with 1,115 IgAN patients in China, in which renal survival rates after diagnosis with IgAN were 83 at 10 years, 74 at 20 years, and 64 % at 30 years. In contrast to the actuarial renal survival rate of 80–90 % for 10 years reported in the literature [
31,
34–
36], this patient experienced rapid deterioration of renal function within only 9 years from the initial clinical diagnosis to dialysis. Thus, the prognosis is extraordinarily poor.
On the other hand, studies on factors enabling prediction of the prognosis of IgAN commonly suggested “unfavorable histopathologic findings” such as glomerular sclerosis and interstitial fibrosis, and “severe proteinuria at presentation,” “arterial hypertension at presentation and during follow-up,” and “elevated serum creatinine at presentation” as strong clinical predictors [
31,
37–
39]. The patient in this case did not have confirmation by biopsy until 8 years after the first onset of symptoms. Since biopsy was performed when histological damages had already progressed significantly, it is difficult to predict the prognosis based on the time points of first onset and first diagnosis based on biopsy findings. Meanwhile, this patient had a serum Cr level of 1.3 mg/dL at the time of initial clinical diagnosis, corresponding to within the normal range, and 266 mg/day of 24 h urine protein, showing that he was not in a severe condition. Furthermore, the patient had neither underlying diseases such as hypertension and diabetes from the past, nor hypertension at the time of diagnosis. In other words, although the patient was in a condition without known strong clinical predictors, the deterioration of his renal function rapidly progressed, unlike the natural progression reported in other studies [
31,
34,
36]. In addition, this decline of renal function overlapped with the patient’s period of exposure to dichloromethane between March 2005 and June 2009 (Fig.
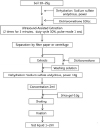
1).
In summary, based on a literature review of the natural history of IgAN, the present case has unusual clinical characteristics such as the age of disease onset, speed of progression, and the prognostic pattern. The authors surmised that the unusual characteristics of this IgAN case provide supporting evidence for the relevance of occupational solvent exposure with IgAN and/or its progression.
This case study has several limitations. First, exposure assessment for toluene could not be conducted because there were almost no relevant data remains. The first episode of hematuria, the initial clinical manifestation of IgAN in this patient, actually began after he had been exposed to toluene while performing analysis of dioxin contents in exhaust gas from an incineration facility during a period of 2 years and 7 months (Table
1). According to reports by the patient and co-workers, however, there was no proper ventilator system in the pretreatment room at that time, and workers usually did not wear protective equipment. Thus, it is presumed that there was direct exposure to a high concentration of toluene. Therefore, the authors cautiously suggest that possibilities of a causal relationship between toluene exposure and the onset and/or aggravation of IgAN could not be ruled out in present case. Secondly, despite the attempt to minimize errors by restoration of the past actual working environment at the site of investigation for exposure, there may still be errors in perfect reflection of the past working environment for the patient including ventilation conditions and the circulation of worker’s traffic. Thirdly, the health effects of other chemical substances used in the laboratory where the patient worked, or potential secondary health effects generated while various chemicals were used in combination with organic solvents, were not evaluated. In addition, another drawback in this case report is that biological monitoring markers for dichloromethane such as blood levels of carboxyhemoglobin (COHb) or urinary dichloromethane [
40,
41] were not evaluated in the exposure assessment.










 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download