Abstract
Purpose
Materials and Methods
Results
Figures and Tables
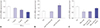 | Fig. 1mRNA expression changes in miR-27b in TNF-α-stimulated MH7A cells. (A) mRNA expression of miR-27b was decreased in MH7A cells stimulated by TNF-α in a concentration-dependent manner. (B) miR-27b mimics upregulates the expression of miR-27b in MH7A cells. (C) miR-27b mimics reduces the decline of miR-27b expression caused by TNF-α stimulation. *p<0.01 vs. ctrl group, †p<0.01 vs. TNF-α+miR-27b mimics NC group. |
 | Fig. 2The effects of TNF-α and miR-27b mimics on the proliferation of MH7A cells were determined by MTT assay. *p<0.01, TNF-α group vs. Ctrl group, †p<0.01, TNF-α+miR-27b mimic group vs. TNF-α+miR-27b mimics NC group. |
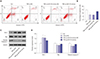 | Fig. 3Upregulation of miR-27b promotes apoptosis of TNF-α-stimulated MH7A cells. (A) The apoptosis of MH7A cells were determined by flow cytometry. (B) The percentage of apoptosis cells in (A). (C) Protein expression of apoptosis-related proteins was determined by Western blot. (D) Quantification of the relative expression levels of apoptosis-related proteins in (C). *p<0.01 vs. ctrl group, †p<0.01 vs. TNF-α+miR-27b mimics NC group. |
 | Fig. 4IL-1β is a target gene of miR-27b. (A) The binding sites between miR-27b and IL-1β predicted by TargetScan. (B) Dual luciferase reporter gene activity was detected to verify the direct combination between miR-27b and IL-1β. *p<0.01 vs. miR-27b mimics NC group. |
 | Fig. 5The effect of miR-27b upregulation on the expression of IL-1β. (A) IL-1β mRNA expression levels in MH7A cells with different treatment were determined by q-PCR. (B) The protein expression levels of IL-1β in MH7A cells with different treatment were determined by Western blot. *p<0.01 vs. ctrl group, †p<0.01 vs. TNF-α+miR-27b mimics NC group. |
 | Fig. 6The effect of miR-27b upregulation on the expression of NF-κB signaling pathway-related proteins was examined by Western blot. (A) The protein expression levels of p-NF-κB p65, NF-κB p65, p-IκBα, and IκBα were determined by Western blot. (B) Quantification of the relative protein expression levels in (A). *p<0.01 vs. ctrl group, †p<0.01 vs. TNF-α+miR-27b mimics NC group. |
ACKNOWLEDGEMENTS
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: Shangwen Lei and Jianying He.
Data curation: Shangwen Lei and Guanghua Chen.
Formal analysis: Shangwen Lei and Liang Deng.
Funding acquisition: Liang Deng and Jianying He.
Investigation: Shangwen Lei, Guanghua Chen, and Liang Deng.
Methodology: Shangwen Lei and Guanghua Chen.
Project administration: Jianying He.
Resources: Shangwen Lei.
Software: Liang Deng.
Supervision: Jianying He.
Validation: Shangwen Lei.
Visualization: Jianying He.
Writing—original draft: Shangwen Lei.
Writing—review & editing: Jianying He.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download