INTRODUCTION
Allergen-specific immunotherapy (AIT) is known to be the only therapeutic modality to alter the natural course of allergic diseases such as
Hymenoptera sting hypersensitivity, allergic rhinitis, and asthma.
1 However, at least 3 years of treatment is recommended to achieve the long-term effect.
2 Discontinuation rate of immunotherapy has been quite variable in individual studies, with subcutaneous immunotherapy ranging from 6% to 84%, and sublingual immunotherapy ranging from 21% to 93%.
3 Therefore, detection and correction of factors contributing to immunotherapy non-adherence are crucial for maximizing the efficacy of immunotherapy.
Adherence is defined as “the extent to which patients follow the instructions they are given for prescribed treatments.”
4 Adherence is preferred over compliance due to the passive and judgmental connotation of the latter.
5 Non-adherence determinants are comprised of patient factors, disease characteristics, treatment regimens or their complexity, and health care systems.
67 A few studies have reported reasons for immunotherapy non-adherence, which were mostly linked with inconvenience, adverse reactions, and cost.
5 Although much effort has been made to improve immunotherapy adherence through education of knowledge about allergic disease and immunotherapy as well as strict follow-up, low adherence still remains a big obstacle for health care providers in AIT practice.
89
Given that adherence is a consequence of complex interactions among numerous variables, it is necessary for each country or region to determine which factors are associated with immunotherapy non-adherence. Therefore, this study aimed to examine the factors affecting immunotherapy non-adherence in real-world practice.
Go to :

MATERIALS AND METHODS
Study population
Retrospective review of electronic medical records was conducted at a single tertiary center, Ajou University Hospital, in South Korea. All subjects received subcutaneous immunotherapy between January 2007 and August 2014. Their diagnoses were allergic rhinitis, bronchial asthma, or atopic dermatitis. Allergen extracts used for immunotherapy were house dust mites (HDMs) as well as tree, grass, or weed pollens, which were determined by physicians through allergen skin prick test or serum specific IgE test using immunoCAP system (ThermoFisher Scientific, Uppsala, Sweden), and clinical relevance. Patients who received sublingual immunotherapy and those who were diagnosed with Hymenoptera sting hypersensitivity or food allergy were excluded from this study. In addition, patients referred to other hospital were not included in this study, since their continuation of AIT was uncertain. This study was approved by the Ethical Review Board of Ajou University (AJIRBMED-MDB-17-502).
Immunotherapy
Immunotherapy consisted of the initial build-up phase and subsequent maintenance phase. For patients who wanted to reduce the duration of build-up phase, accelerated schedules were implemented: rush, cluster, or ultra-rush immunotherapy. For rush immunotherapy, patients were admitted for 3 to 4 days, and were injected with gradually increasing concentrations and doses of allergen extracts. For cluster immunotherapy, patients were injected with allergen extracts 2 to 3 times a day for 4 to 6 weeks. For ultra-rush immunotherapy, build-up phase was reduced to 2 days, and patients were injected at shorter intervals and with rapidly increasing concentrations and doses of allergen extracts. For conventional build-up immunotherapy, allergen extracts were administered once a week for 2 to 3 months during build-up phase. During maintenance phase, the same concentration of allergen extracts was given at 4 week intervals, regardless of the build-up methods for immunotherapy. Allergen extracts used in this study were L-tyrosine adsorbed Tyrosine S® (Allergy Therapeutics, Worthing, UK) or aluminum hydroxide adsorbed Novo-Helisen® (Allergopharma, Reinbeck, Germany).
Study outcomes
Adherence was determined at the completion of 3 years of AIT. If patients continued to receive AIT for at least 3 years, they were deemed to be adherent. Non-adherence was defined as discontinuation of AIT before 3 years.
Patient characteristics and immunotherapy-related factors were collected to find an association between non-adherence and immunotherapy. For patient factors, we collected data on age, sex, diagnosis of allergic diseases and its duration, non-allergic comorbid diseases such as hypertension, cardiac disease, or diabetes mellitus, follow-up visit to other departments in the same hospital, and distance between hospital and patients' residence. Age was classified into three categories: <20 years, 20 to 40 years, and >40 years. Allergic asthma and rhinitis were combined into respiratory allergy. Disease duration was divided into three groups: <5 years, 5–10 years, and >10 years. Distance between hospital and patients' residence was divided into three groups according to their addresses documented in the medical chart: 1) inside city, 2) inside province, or 3) outside province.
For immunotherapy factors, information about the types of allergen extracts used for immunotherapy, type of build-up schedule, and pharmaceutical companies were collected. Specific IgE level to
Dermatophagoides farinae (
Df) of ≥17.5 kU/L has been shown to be related to favorable clinical response to immunotherapy.
10 Therefore, to evaluate whether this specific IgE level is correlated with non-adherence, patients were divided into two categories: those with <17.5 kU/L and those with ≥17.5 kU/L.
Statistical analysis
All data were converted into categorical variables, as mentioned earlier in the Materials and Methods section. To determine whether clinical variables are correlated with immunotherapy non-adherence, a generalized linear model was used in univariate analysis. Odds ratios (ORs) are presented with 95% confidence intervals (CIs). To analyze changes in the cumulative proportion of subjects to continue AIT over time, Kaplan-Meier curves were employed. Multivariate logistic regression analysis was applied to find independent variables to be associated with immunotherapy non-adherence. Variable selection was made, considering p value derived from univariate analysis and multicollinearity between variables. All statistical analysis was performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA) and SAS 9.4 (SAS Institute Inc., Cary, NC, USA). p value less than 0.05 was considered statistically significant.
Go to :

RESULTS
A total of 1162 patients were enrolled in this study. The mean age was 32.6±13.7 years, and there were 593 (51.0%) female patients. The numbers of patients treated with immunotherapy for respiratory allergy and atopic dermatitis were 858 (73.8%) and 304 (26.2%), respectively. While patients treated with Tyrosine S® mainly had atopic dermatitis (88.6%), most of the patients treated with Novo-Helisen® had respiratory allergy (77.8%). The starting age of immunotherapy and the number of patients in age categories by 10-year age groups showed normal distributions (
Fig. 1). Most patients were in their 30s (25.9%). More data on patient characteristics and immunotherapy-related factors are shown in
Table 1.
 | Fig. 1Starting age of immunotherapy in age categories by 10-year age groups.
|
Table 1
Demographic and Clinical Characteristics of Study Patients
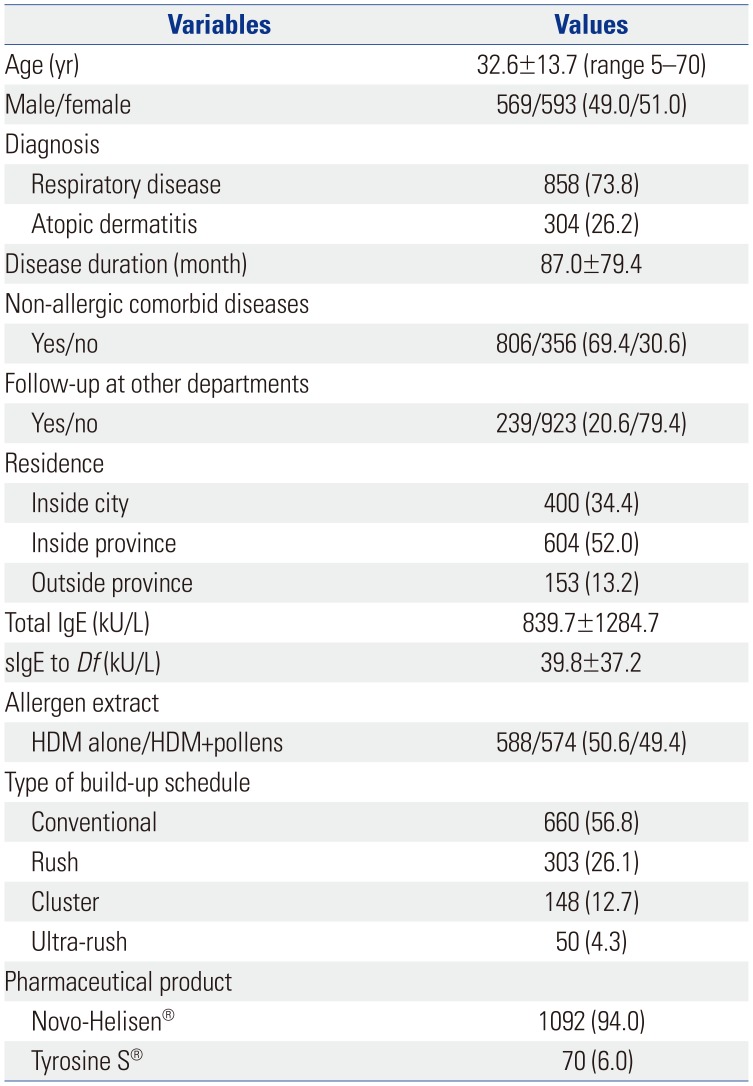
|
Variables |
Values |
|
Age (yr) |
32.6±13.7 (range 5–70) |
|
Male/female |
569/593 (49.0/51.0) |
|
Diagnosis |
|
|
Respiratory disease |
858 (73.8) |
|
Atopic dermatitis |
304 (26.2) |
|
Disease duration (month) |
87.0±79.4 |
|
Non-allergic comorbid diseases |
|
|
Yes/no |
806/356 (69.4/30.6) |
|
Follow-up at other departments |
|
|
Yes/no |
239/923 (20.6/79.4) |
|
Residence |
|
|
Inside city |
400 (34.4) |
|
Inside province |
604 (52.0) |
|
Outside province |
153 (13.2) |
|
Total IgE (kU/L) |
839.7±1284.7 |
|
sIgE to Df (kU/L) |
39.8±37.2 |
|
Allergen extract |
|
|
HDM alone/HDM+pollens |
588/574 (50.6/49.4) |
|
Type of build-up schedule |
|
|
Conventional |
660 (56.8) |
|
Rush |
303 (26.1) |
|
Cluster |
148 (12.7) |
|
Ultra-rush |
50 (4.3) |
|
Pharmaceutical product |
|
|
Novo-Helisen®
|
1092 (94.0) |
|
Tyrosine S®
|
70 (6.0) |

Non-adherence rate was 19.6% (
Fig. 2A). Cumulative proportion of non-adherent subjects over time was analyzed by Kaplan-Meier curve analysis. A gradual decrease in the discontinuation rate of immunotherapy appeared without abrupt dropout (
Fig. 2B). The mean treatment duration was 6.7±3.1 years in adherent group, while it was 1.6±0.9 years in non-adherent group (
p<0.001).
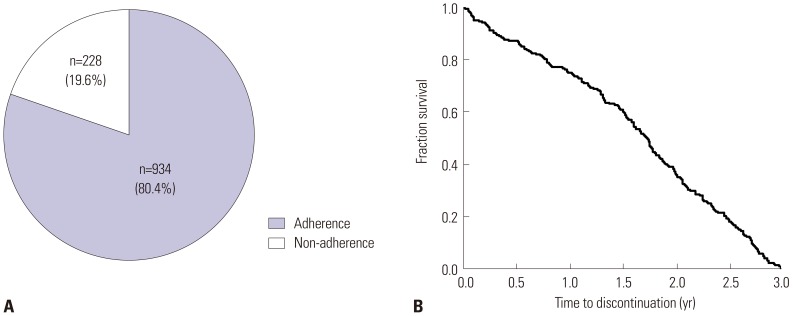 | Fig. 2Proportion of adherence and non-adherence to immunotherapy among study subjects (A) and cumulative proportion of immunotherapy in non-adherent patients over time (B).
|
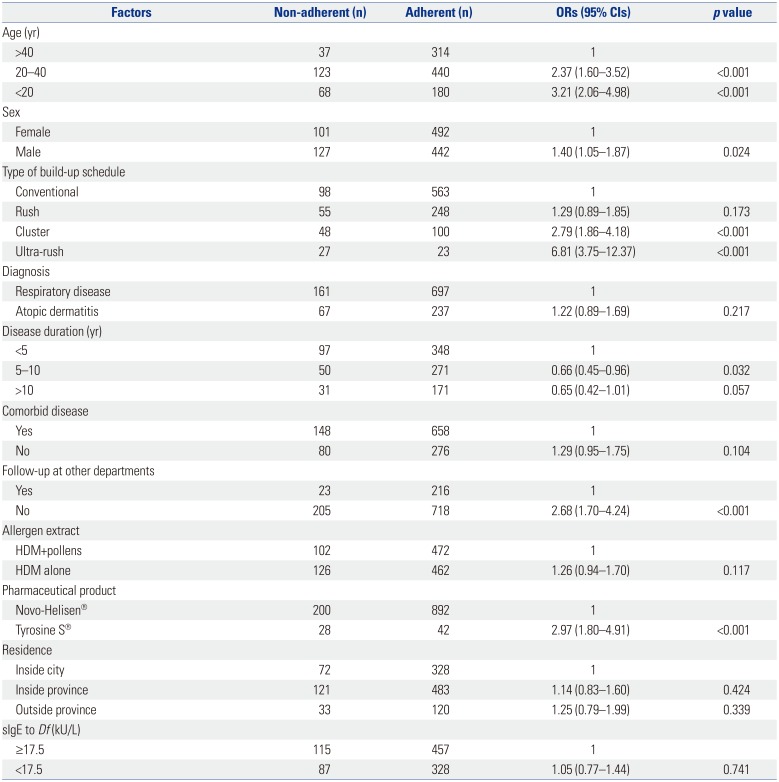
Univariate analysis
Patients aged <20 years (OR 3.21, 95% CI 2.06–4.98) and 20–40 years (OR 2.37, 95% CI 1.60–3.52) were more likely to be non-adherent than those aged >40 years (
Table 2). Male subjects were more likely to be non-adherent than female subjects (OR 1.40, 95% CI 1.05–1.87). Patients with accelerated build-up schedule were more non-adherent: rush (OR 1.29, 95% CI 0.89–1.85), cluster (OR 2.79, 95% CI 1.86–4.18), and ultra-rush immunotherapy (OR 6.81, 95% CI 3.75–12.37) compared to those receiving conventional build-up immunotherapy. No visit to other departments in the same hospital was associated with non-adherence (OR 2.68, 95% CI 1.70–4.24). Adherence rates were analyzed among the pharmaceutical companies of allergen extracts. Patients treated with Tyrosine S® were more likely to be non-adherent than those treated with Novo-Helisen® (OR 2.97, 95% CI 1.80–4.91).
Table 2
Comparisons of Factors Affecting Immunotherapy Non-Adherence between Non-Adherent and Adherent Groups in Univariate Analysis
|
Factors |
Non-adherent (n) |
Adherent (n) |
ORs (95% CIs) |
p value |
|
Age (yr) |
|
|
|
|
|
>40 |
37 |
314 |
1 |
|
|
20–40 |
123 |
440 |
2.37 (1.60–3.52) |
<0.001 |
|
<20 |
68 |
180 |
3.21 (2.06–4.98) |
<0.001 |
|
Sex |
|
|
|
|
|
Female |
101 |
492 |
1 |
|
|
Male |
127 |
442 |
1.40 (1.05–1.87) |
0.024 |
|
Type of build-up schedule |
|
|
|
|
|
Conventional |
98 |
563 |
1 |
|
|
Rush |
55 |
248 |
1.29 (0.89–1.85) |
0.173 |
|
Cluster |
48 |
100 |
2.79 (1.86–4.18) |
<0.001 |
|
Ultra-rush |
27 |
23 |
6.81 (3.75–12.37) |
<0.001 |
|
Diagnosis |
|
|
|
|
|
Respiratory disease |
161 |
697 |
1 |
|
|
Atopic dermatitis |
67 |
237 |
1.22 (0.89–1.69) |
0.217 |
|
Disease duration (yr) |
|
|
|
|
|
<5 |
97 |
348 |
1 |
|
|
5–10 |
50 |
271 |
0.66 (0.45–0.96) |
0.032 |
|
>10 |
31 |
171 |
0.65 (0.42–1.01) |
0.057 |
|
Comorbid disease |
|
|
|
|
|
Yes |
148 |
658 |
1 |
|
|
No |
80 |
276 |
1.29 (0.95–1.75) |
0.104 |
|
Follow-up at other departments |
|
|
|
|
|
Yes |
23 |
216 |
1 |
|
|
No |
205 |
718 |
2.68 (1.70–4.24) |
<0.001 |
|
Allergen extract |
|
|
|
|
|
HDM+pollens |
102 |
472 |
1 |
|
|
HDM alone |
126 |
462 |
1.26 (0.94–1.70) |
0.117 |
|
Pharmaceutical product |
|
|
|
|
|
Novo-Helisen®
|
200 |
892 |
1 |
|
|
Tyrosine S®
|
28 |
42 |
2.97 (1.80–4.91) |
<0.001 |
|
Residence |
|
|
|
|
|
Inside city |
72 |
328 |
1 |
|
|
Inside province |
121 |
483 |
1.14 (0.83–1.60) |
0.424 |
|
Outside province |
33 |
120 |
1.25 (0.79–1.99) |
0.339 |
|
sIgE to Df (kU/L) |
|
|
|
|
|
≥17.5 |
115 |
457 |
1 |
|
|
<17.5 |
87 |
328 |
1.05 (0.77–1.44) |
0.741 |

Adherence of patients with atopic dermatitis was not different from that of patients with respiratory allergy (OR 1.22, 95% CI 0.89–1.69). Non-adherence in patients with disease duration of 5–10 years (OR 0.66, 95% CI 0.45–0.96) and >10 years (OR 0.65, 95% CI 0.42–1.01) were lesser than that of patients with disease duration of less than 5 years. The absence of non-allergic comorbid diseases was not related with non-adherence (OR 1.29, 95% CI 0.95–1.75). Patients treated with HDM allergen extracts alone tended to be more non-adherent than those treated with HDMs plus pollens, although the difference was not statistically significant (OR 1.26, 95% CI 0.94–1.70). There was no significant difference in non-adherence between patients living inside province (OR 1.14, 95% CI 0.83–1.60) and those living outside province (OR 1.25, 95% CI 0.79–1.99) compared to those living inside city.
Initial total IgE levels were not different between adherent and non-adherent groups (861.0±1320.2 kU/L vs. 755.4±1132.4 kU/L, p=0.227). Also, serum specific IgE levels to Df were not different between adherent and non-adherent groups (39.8±37.3 kU/L vs. 39.8±37.0 kU/L, p=0.984). Moreover, the difference in adherence was not observed between patients with initial higher (≥17.5 kU/L) and lower IgE levels (<17.5 kU/L) to Df (OR 1.05, 95% CI 0.77–1.44).
Cumulative proportion of patients who continue immunotherapy over time was analyzed using Kaplan-Meier curves. Patients aged <20 years (
p<0.001) and 20–40 years (
p<0.001) discontinued immunotherapy earlier than those aged >40 years (
Fig. 3A). Immunotherapy continuation rate was lower in male patients than in female patients (
p=0.022) (
Fig. 3B). Continuation rate was lower in patients receiving cluster (
p<0.001) and ultra-rush build-up immunotherapy (
p<0.001) compared to those receiving conventional build-up immunotherapy; however, patients receiving rush build-up immunotherapy were not different during 3-year immunotherapy (
Fig. 3C). Patients with disease duration of less than 5 years discontinued earlier than those with 5–10 years and >10 years (
p=0.026) (
Fig. 3D). Patients receiving Tyrosine S® extracts discontinued immunotherapy earlier than those receiving Novo-Helisen® extracts (
p<0.001) (
Fig. 3E). Patients without visit to other departments in the same hospital discontinued immunotherapy earlier than those with (
p<0.001) (
Fig. 3F). However, the continuation rate was not different between patients with higher (≥17.5 kU/L) and lower initial IgE levels to
Df (<17.5 kU/L) patients (
p=0.755).
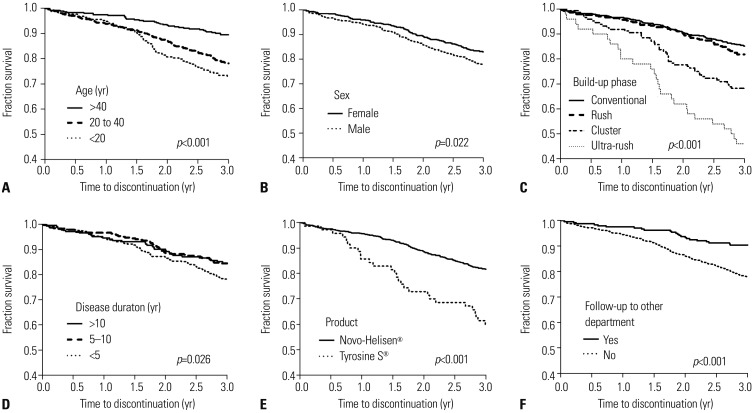 | Fig. 3Proportion of patients on immunotherapy over time by Kaplan-Meier analysis regarding age (A), sex (B), type of build-up schedule (C), disease duration (D), pharmaceutical product (E), and follow-up at other departments (F).
|
Multivariate analysis
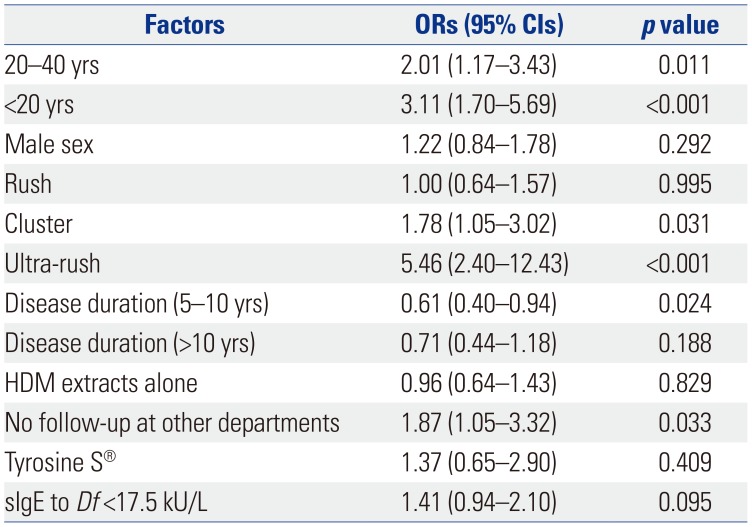
Patients aged <20 years (OR 3.11, 95% CI 1.70–5.69) and 20–40 years (OR 2.01, 95% CI 1.17–3.43) were more likely to be non-adherent than those aged >40 years (
Table 3). As for the type of build-up schedule, patients receiving cluster (OR 1.78, 95% CI 1.05–3.02) and ultra-rush immunotherapy (OR 5.46, 95% CI 2.40–12.43) were more likely to be non-adherent than those receiving conventional build-up immunotherapy. Patients with disease duration of less than 5 years were more non-adherent than those with 5–10 years (OR 0.61, 95% CI 0.40–0.94) and >10 years (OR 0.71, 95% CI 0.44–1.18). Also, no visit to other departments in the same hospital was associated with non-adherence (OR 1.87, 95% CI 1.05–3.32).
Table 3
Factors Affecting Immunotherapy Non-Adherence in Multivariate Analysis
|
Factors |
ORs (95% CIs) |
p value |
|
20–40 yrs |
2.01 (1.17–3.43) |
0.011 |
|
<20 yrs |
3.11 (1.70–5.69) |
<0.001 |
|
Male sex |
1.22 (0.84–1.78) |
0.292 |
|
Rush |
1.00 (0.64–1.57) |
0.995 |
|
Cluster |
1.78 (1.05–3.02) |
0.031 |
|
Ultra-rush |
5.46 (2.40–12.43) |
<0.001 |
|
Disease duration (5–10 yrs) |
0.61 (0.40–0.94) |
0.024 |
|
Disease duration (>10 yrs) |
0.71 (0.44–1.18) |
0.188 |
|
HDM extracts alone |
0.96 (0.64–1.43) |
0.829 |
|
No follow-up at other departments |
1.87 (1.05–3.32) |
0.033 |
|
Tyrosine S®
|
1.37 (0.65–2.90) |
0.409 |
|
sIgE to Df <17.5 kU/L |
1.41 (0.94–2.10) |
0.095 |

Male sex (OR 1.22, 95% CI 0.84–1.78), rush immunotherapy (OR 1.00, 95% CI 0.64–1.57), HDM extracts alone (OR 0.96, 95% CI 0.64–1.43), the allergen product Tyrosine S® (OR 1.37, 95% CI 0.65–2.90), and specific IgE to Df <17.5 kU/L (OR 1.41, 95% CI 0.94–2.10) were not found to be associated with immunotherapy non-adherence in multivariate analysis.
Go to :

DISCUSSION
In this study, non-adherence rate was 19.6%, and the following factors were found to be associated with immunotherapy non-adherence: younger age less than 40 years, cluster and ultra-rush build-up schedules, disease duration of less than 5 years, and no visit to other departments in the same hospital.
Previous studies have investigated whether age and sex would be adherence factors for immunotherapy. Although some studies have demonstrated discrepancies regarding age factor for immunotherapy adherence,
11 others have shown that older age is a significant factor for immunotherapy adherence.
1213 A study conducted in the United States reported that immunotherapy adherence increased with age: 46.7% (18 to 35 years), 58.3% (36 to 65 years), and 78.7% (older than 66 years),
14 while another study showed that the highest dropout rate was observed in the age group of 16 to 25 years, and the lowest dropout rate was observed in the age group of ≥40 years.
13 Moreover, the middle-aged group (18 to 45 years) were more non-adherent than younger (<18 years) and older (>45 years) age groups,
12 which is different from our results. In Korea, most patients in the younger age group consist of middle and high school students whose school hours overlap with clinic hours in hospitals, so the students spend relatively more time studying in school. In the present study, males were non-adherent in univariate analysis, which was not significant in multivariate analysis. A previous study reported that males were more non-adherent than females.
13 The difference between males and females may be attributed to the active social performance of male subjects, which can be different among countries or regions. Taken together, it is thought that the results reflect active participation of those age and sex groups in social activity, resulting in low immunotherapy adherence.
Rush immunotherapy schedule was found to be associated with non-adherence in a previous study.
12 Although adherence of patients receiving rush schedule were not different from that of patients receiving conventional schedule, other accelerated schedules, such as cluster and ultra-rush schedules, were closely associated with non-adherence in the present study. Accelerated schedules lead to an increase in the incidence of rate of systemic reactions predominantly during build-up phase.
10 However, based on the result from the cumulative proportion of patients who continued immunotherapy, immunotherapy discontinuation did not mainly occur early within build-up phase, but evenly occurred, suggesting that low adherence to accelerated schedules may not have been attributed to the increasing incidence of systemic reactions. The reason for discontinuing immunotherapy was not directly related to adverse reactions to immunotherapy.
15 Accelerated schedules are usually employed by patients who do not have enough time to receive a longer conventional schedule. Therefore, it can be postulated that accelerated schedules are most likely to be chosen by subjects with active social performance, whose discontinuation rate increases due to their insufficient time.
Shorter disease duration less than 5 years was found to be a non-adherent factor. Association between disease duration and adherence of immunotherapy has not been investigated until now. Disease duration is a complicated factor in terms of adherence. Longer disease duration has been considered to be associated with non-adherence in chronic disease.
16 However, there were conflicting results. For example, non-adherence to medication was more likely in those with shorter disease duration in inflammatory bowel disease.
17 Patients with shorter disease duration might not have an information enough to maintain immunotherapy, while those with longer disease duration are likely to be exposed to the information of allergic disease as a chronic nature and the benefit of immunotherapy as an only disease modifying treatment option over time. In addition, patients with longer disease duration are likely to obviously experience lack of efficacy and only symptomatically benefit from medication, which can make them adhere more to immunotherapy.
Among patient-related factors, no visit to other departments in the same hospital was correlated with non-adherence; however, patients' residence and non-allergic comorbid diseases were not associated with adherence. In a previous study, patients with psychiatric diseases showed a higher level of adherence than those without,
14 since their comorbid psychiatric diseases can make them visit hospitals regularly. In the present study, patients with visit to other departments in the same hospital where immunotherapy was prescribed were more likely to be adherent than those without. Patients' residence did not affect adherence as in other studies.
14 With advances in the transportation system, distance from hospital may not prevent patients from visiting distant hospitals.
Specific IgE to
Df was analyzed whether it would be significantly associated with non-adherence. In a previous study, patients with lower IgE to
Df (<17.5 kU/L) was found to have poorer efficacy in immunotherapy than those with higher IgE (≥17.5 kU/L).
10 However, specific IgE was found not to be associated with adherence, regardless of higher (≥17.5 kU/L) and lower (<17.5 kU/L) specific IgE levels in this study.
A few studies were conducted to examine whether the type of allergen extracts for immunotherapy would be correlated with immunotherapy adherence; however, their results were inconsistent with one another. In a previous study, single allergen immunotherapy has been reported to be a predictor of premature discontinuation;
18 however, in other studies, the types of allergen extracts (HDMs extracts alone and HDMs plus pollens extracts) were not related to immunotherapy adherence, which was in agreement with the results of our study.
11 The kind of allergic diseases showed different influence on immunotherapy adherence.
1119 Patients with both asthma and rhinitis were more adherent than those with either of them.
20 However, the kind of respiratory allergic diseases was not correlated with adherence in a previous study.
12 Additionally, we attempted to determine whether atopic dermatitis (AD) is correlated with immunotherapy adherence. Therapeutic efficacy of immunotherapy in AD has been shown to be low.
21 In addition, a considerable number of patients with AD experience aggravation of their eczema or pruritus during immunotherapy.
22 An accurate proportion of patients experiencing clinical improvement and adverse reactions has not yet been elucidated. In the present study, immunotherapy adherence in patients with AD was not different from that in those with respiratory allergic disease. Therefore, it is conceivable that the low efficacy of immunotherapy and incidence of adverse reactions may not affect immunotherapy adherence in patients with AD.
There were some limitations in this study. First, we should have asked patients about their reasons for discontinuing immunotherapy. Secondly, this was a retrospective study. Prospective studies are needed to examine patient-related factors, such as health and medical expenses. Thirdly, our definition of adherence may differ from those of other studies, which can lead to difficulty in comparing factors associated with immunotherapy adherence between their results and ours.
In conclusion, various factors are related to immunotherapy adherence affecting the utility of immunotherapy. Clinicians should be aware of the factors associated with immunotherapy non-adherence in individual patients to maximize the utility of allergen specific subcutaneous immunotherapy. In addition, the definition of adherence and non-adherence to immunotherapy should be addressed in future immunotherapy guidelines.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download