Abstract
Purpose
The prevalence and clinical outcomes of asymptomatic carotid artery stenosis (CAS) in patients with coronary artery disease (CAD) have not been thoroughly studied. We examined the prevalence and predictors of asymptomatic CAS detected by carotid angiography and determined the impact of concomitant CAS on prognosis in patients undergoing coronary angiography (CAG) due to CAD.
Materials and Methods
Between January 2013 and July 2015, 395 patients who underwent carotid digital subtraction angiography to screen for CAS during CAG were analyzed. The presence of CAS was defined as angiographically significant stenosis (≥50%). Major adverse cardiac and cerebrovascular event (MACCE) rates were compared between patients with and without CAS. MACCEs included a composite of cardiac death, cerebrovascular death, acute myocardial infarction, and stroke.
Results
Of the 395 patients, 101 (25.5%) patients had significant CAS. The independent predictors of CAS were age, male sex, hypertension, diabetes, and multi-vessel disease. In patients with CAD, the presence of CAS was as an independent predictor for MACCEs after adjusting for confounding factors (hazard ratio 2.47, 95% confidence interval 1.16–5.24, p=0.018).
Conclusion
Asymptomatic CAS was documented in up to 25% of patients with CAD. The presence of CAS in patients with CAD was associated with a higher rate of MACCEs. Therefore, detection of CAS by carotid angiography during CAG may be important for risk stratification for CAD patients, particularly those with multi-vessel disease.
According to the World Health Organization, the world's first and second highest causes of mortality in 2016 were coronary artery disease (CAD) and stroke. Because both diseases result from preexisting atherosclerosis that remains asymptomatic over a long period of time,1 it is difficult to prevent the first cardiovascular event. Carotid artery stenosis (CAS) is a common cause of stroke and can easily be assessed by readily available tests. A number of previous studies have demonstrated a correlation between CAD and CAS, as they share similar risk factors.23 Thus, it is important to identify the risk for CAS or stroke in patients with CAD.
There are several ways to detect CAS, including ultrasound of carotid intima media thickness, computed tomography (CT), or catheter angiography. Compared to ultrasound and CT, catheter angiography results in an excellent image and provides additional anatomical details, including the percentage of stenosis, the location of the bifurcation in relation to the angle of the jaw, the extent of plaque, and the status of contralateral carotid and collateral flow.4
Nevertheless, most studies have evaluated carotid artery images obtained by ultrasound or CT. We examined the prevalence and predictors of asymptomatic CAS detected by carotid angiography and evaluated the impact of concomitant CAS on prognosis for patients undergoing coronary angiography (CAG) because of angina.
Between January 2013 and July 2015, a total of 395 patients who underwent carotid digital subtraction angiography (DSA) to screen for CAS during CAG at Sanggye Paik Hospital in Seoul, South Korea were analyzed. The cohort comprised consecutive patients who underwent CAG by two of five interventionists during the period, and this accounted for 30% of all patients in the center during the period. All patients underwent CAG because of stable angina, unstable angina, or myocardial infarction.
In case of a trans-right radial artery approach, after diagnostic angiography of the left coronary artery with a 4 Fr Judkins left catheter, we located the tip of the catheter at the ostium of the left common carotid artery. DSA projection was performed at a left anterior oblique angle of 60°. A right carotid angiogram was performed using a 4 Fr Judkins right catheter at a right anterior oblique angle of 60°. A total of 12 cc contrast was used on a shot of 6 cc. We used a Judkin right catheter only when we applied a left radial approach and both femoral approaches.
We evaluated the degree of stenosis of the carotid artery using the North American Symptomatic Carotid Endarterectomy Trial (NASCET) method, which compares stenosis to the distal normal post-stenotic internal carotid artery diameter.5
The presence of significant CAS was defined as ≥50% stenosis on the DSA image. Maximum stenosis was defined as the greatest stenosis observed in the common carotid artery and bifurcation of the internal carotid artery, and the severity of CAS was classified as 1) moderate stenosis (<70%) or 2) severe stenosis (≥70%). If carotid artery angiography showed ≥50% stenosis, we performed another angiography at other angle for accurate evaluation. CAD was defined as angiographically significant stenosis (≥50%) of the coronary arteries, and the severity of CAD was classified as minimal and one-, two-, or three-vessel disease.
The major adverse cardiac and cerebrovascular event (MACCE) rate was compared between patients with and without CAS. MACCE included a composite of cardiac death, cerebrovascular death, acute myocardial infarction, and stroke during the follow-up period. If the patient had multiple events, we included the first event in MACCE. The secondary endpoint was major adverse cardiac events (MACE), which included cardiac death, myocardial infarction, and target vessel revascularization (TVR).
The Statistical Package of Social Science for Window, release 25.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses. The t test was used to compare continuous variables, and the chi-square test was used to compare frequency variables. Logistic regression analyses were used to analyze the independent factors of CAS, and Cox proportional analyses were used to evaluate the prognostic factors of MACCEs. In all analyses, a p value <0.05 was considered significant.
From January 2013 to July 2015, a total of 395 patients were enrolled in this study, and the mean follow-up duration was 23 months. Baseline characteristics according to the presence of CAS are shown in Table 1. Significant CAS was observed in 101 patients (25.5%). CAS was more common in males and older adults. There were no significant differences according to disease presentation, although multi-vessel disease was significantly more frequent in patients with CAS. Patients with CAS were more likely to have an underlying disease, such as hypertension and diabetes, and to undergo coronary artery bypass surgery. Laboratory findings showed higher high-sensitivity C-reactive protein (hs-CRP) and lower high-density lipoprotein cholesterol (HDL-C) levels in the CAS group than in the non-CAS group.
More than half of patients with CAS have multi-vessel disease, and CAS was confirmed in more than half of all patients with three-vessel disease in this study (Fig. 1). MACCE occurred in 14% of patients in the CAS group (Table 2). Although MACE was also more prevalent in the CAS group, it was not statistically significant. In univariate analysis, factors affecting the presence of CAS were age, male sex, the presence of hypertension, diabetes, and multi-vessel disease. Significance was maintained in multivariate analysis (Table 3).
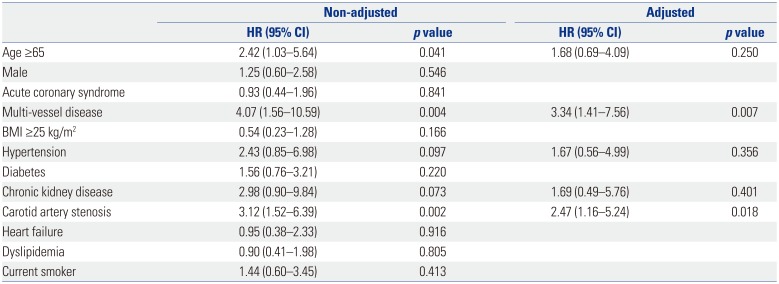
As shown in Table 4, CAS and multi-vessel disease were independent factors associated with MACCEs [hazard ratio (HR) 2.47, 95% confidence interval (CI) 1.16–5.24, p value 0.018; HR 3.34, 95% CI 1.41–7.56, p value 0.007, respectively]. Kaplan-Meier curves for the 3-year follow-up period also showed statistically significant differences in outcomes depending on the presence or absence of CAS (Fig. 2).
The prevalence of CAS in patients with CAD was 25.5% in our study. This prevalence is not significantly different from the 19.6% reported by carotid ultrasound in CAD patients in Japan.6 Asymptomatic CAS was found in up to 50% of patients with multi-vessel disease and was more prevalent in patients with an underlying disease, including hypertension, diabetes, and chronic kidney disease (CKD). In addition, the presence of CAS was associated with a 2.47-fold higher risk for MACCEs after adjusting for several well-known risk factors, including age, hypertension, and CKD.
CAS causes approximately 10–20% of strokes,4 and early detection of CAS is important for stroke prevention. Moreover, there have been reports that the incidence of CAS increases with CAD. Jeevarethinam, et al.7 reported that carotid plaque and carotid intima-media thickness assessed by ultrasound are associated with CAD. Cappelletti, et al.8 showed a relationship between significant carotid disease and CAD severity. Cohen, et al.2 indicated that carotid disease is associated with CAD, as assessed via CT angiograms. Considering these studies, it is important to confirm the presence of CAS in patients with CAD. However, most previous studies have used carotid ultrasound to detect CAS.
The strength and unique features of our study are that we investigated the relationship between these two diseases based on angiography of the carotid artery during the recording of coronary angiograms. This method has several advantages over carotid ultrasound. First, DSA has higher accuracy than ultrasound in detecting CAS.910 Second, ultrasound can only detect atherosclerosis located in a bifurcation lesion, whereas DSA can be used to obtain not only the bifurcation lesion but also information that can affect treatment options.
Considering the relationship between CAD and CAS, it is reasonable to consider the presence of CAS in patients with CAD. In addition, this strategy could be time-saving for patients and reduce inconvenience with performing additional tests to evaluate CAS. Our center detected approximately 25% of asymptomatically significant CAS patients using this approach. Of these, 50 patients (12.7%) had more than 70% severe lesions. Further intervention, such as carotid stenting or endarterectomy, was considered after cooperation with the neurologist. We also took into consideration the patient's condition. Overall, carotid stenting was performed in 15 patients (30%). During the follow-up period, six patients with CAS developed stroke, three of whom with severe CAS were recommended for carotid stent implantation but refused.
In our study, CAS was associated with older age, a higher prevalence of multi-vessel CAD, hypertension, diabetes, and CKD. In addition, HDL-cholesterol levels were lower and hs-CRP levels were higher in CAS patients than in the non-CAS group. Possible explanations for these observations are as follows: 1) Patients with diabetes, hypertension, and low HDL-cholesterol may develop systemic atherosclerosis due to limited glycemic control, altered shear stress, and/or an impaired cholesterol reuptake mechanism. 2) hs-CRP may enhance the instability of plaques to induce plaque rupture. 3) Patients with multi-vessel artery lesions may be more vulnerable to plaque rupture, which increases the risk for overt atherosclerotic disease. In multivariate analyses, the presence of CAS was associated with older age, sex, hypertension, and diabetes. In addition, multi-vessel disease showed a three-fold incidence of CAS, and the presence of CAS was identified as an independent risk factor for MACCEs. Thus, patients with multiple underlying diseases or multi-vessel disease should be carefully evaluated for CAS.
There were some limitations to this study. First, because our study was a nonrandomized, observational study, selection bias and unmeasured confounding factors could not be eliminated. Second, as a single-center study, patients in our study may not reflect the general population of a large region, and the small sample size may have weakened the statistical power. Patients enrolled by two interventionists were consecutive during the index period; however, there was a possibility of selection bias because we did not target all of the patients in our center. Third, the fact initial screening carotid artery DSA was only one projection, which could lead to underestimation of the arteries that have asymmetrical eccentric stenosis. Fourth, concurrent coronary and carotid angiography leads increased consumption of contrast dye, fluoroscopy time, and increases the probability of contrast induced nephropathy.
In conclusion, asymptomatic CAS was found in up to 25% of patients with CAD. The presence of CAS in patients with CAD was associated with a higher rate of MACCEs. Therefore, the detection of CAS by carotid angiography during CAG may be important for risk stratification of these patients, particularly those with multi-vessel disease.
References
1. Yamagishi M, Terashima M, Awano K, Kijima M, Nakatani S, Daikoku S, et al. Morphology of vulnerable coronary plaque: insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J Am Coll Cardiol. 2000; 35:106–111. PMID: 10636267.

2. Cohen GI, Aboufakher R, Bess R, Frank J, Othman M, Doan D, et al. Relationship between carotid disease on ultrasound and coronary disease on CT angiography. JACC Cardiovasc Imaging. 2013; 6:1160–1167. PMID: 24229768.
3. Akosah KO, McHugh VL, Barnhart SI, Schaper AM, Mathiason MA, Perlock PA, et al. Carotid ultrasound for risk clarification in young to middle-aged adults undergoing elective coronary angiography. Am J Hypertens. 2006; 19:1256–1261. PMID: 17161771.

4. Grotta JC. Clinical practice. Carotid stenosis. N Engl J Med. 2013; 369:1143–1150. PMID: 24047063.
5. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991; 325:445–453. PMID: 1852179.

6. Tanimoto S, Ikari Y, Tanabe K, Yachi S, Nakajima H, Nakayama T, et al. Prevalence of carotid artery stenosis in patients with coronary artery disease in Japanese population. Stroke. 2005; 36:2094–2098. PMID: 16179563.

7. Jeevarethinam A, Venuraju S, Dumo A, Ruano S, Rosenthal M, Nair D, et al. Usefulness of carotid plaques as predictors of obstructive coronary artery disease and cardiovascular events in asymptomatic individuals with diabetes mellitus. Am J Cardiol. 2018; 121:910–916. PMID: 29499924.

8. Cappelletti A, Astore D, Godino C, Bellini B, Magni V, Mazzavillani M, et al. Relationship between Syntax Score and prognostic localization of coronary artery lesions with conventional risk factors, plasma profile markers, and carotid atherosclerosis (CAPP Study 2). Int J Cardiol. 2018; 257:306–311. PMID: 29506713.

9. Anzidei M, Napoli A, Zaccagna F, Di Paolo P, Saba L, Cavallo Marincola B, et al. Diagnostic accuracy of colour Doppler ultrasonography, CT angiography and blood-pool-enhanced MR angiography in assessing carotid stenosis: a comparative study with DSA in 170 patients. Radiol Med. 2012; 117:54–71. PMID: 21424318.

10. Nederkoorn PJ, van der Graaf Y, Hunink MG. Duplex ultrasound and magnetic resonance angiography compared with digital subtraction angiography in carotid artery stenosis: a systematic review. Stroke. 2003; 34:1324–1332.
Fig. 1
Distribution of significant CAS according to the severity of CAD. CAS, carotid artery stenosis; CAD, coronary artery disease.
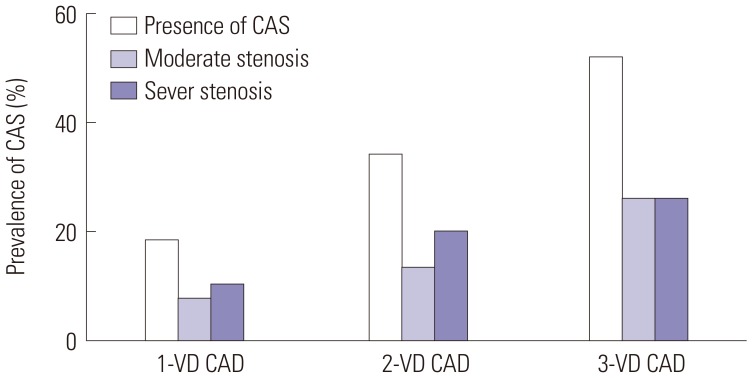
Fig. 2
Clinical outcomes according to the presence of CAS. CAS, carotid artery stenosis; MACCE, major adverse cardiac and cerebrovascular event.
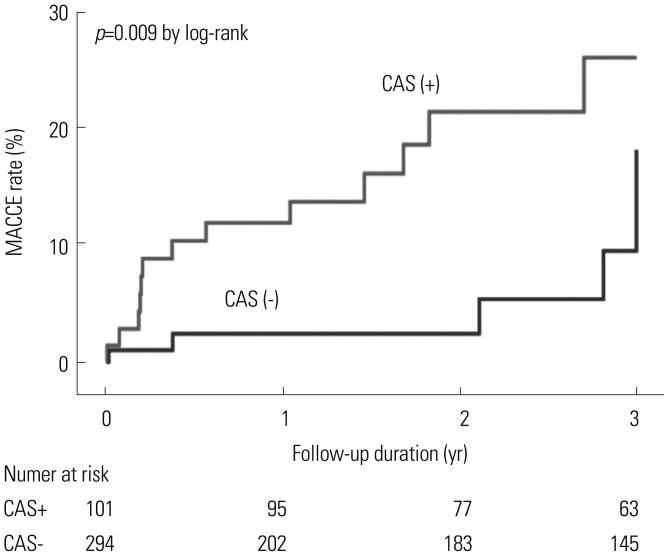
Table 1
Baseline Characteristics according to the Presence of CAS
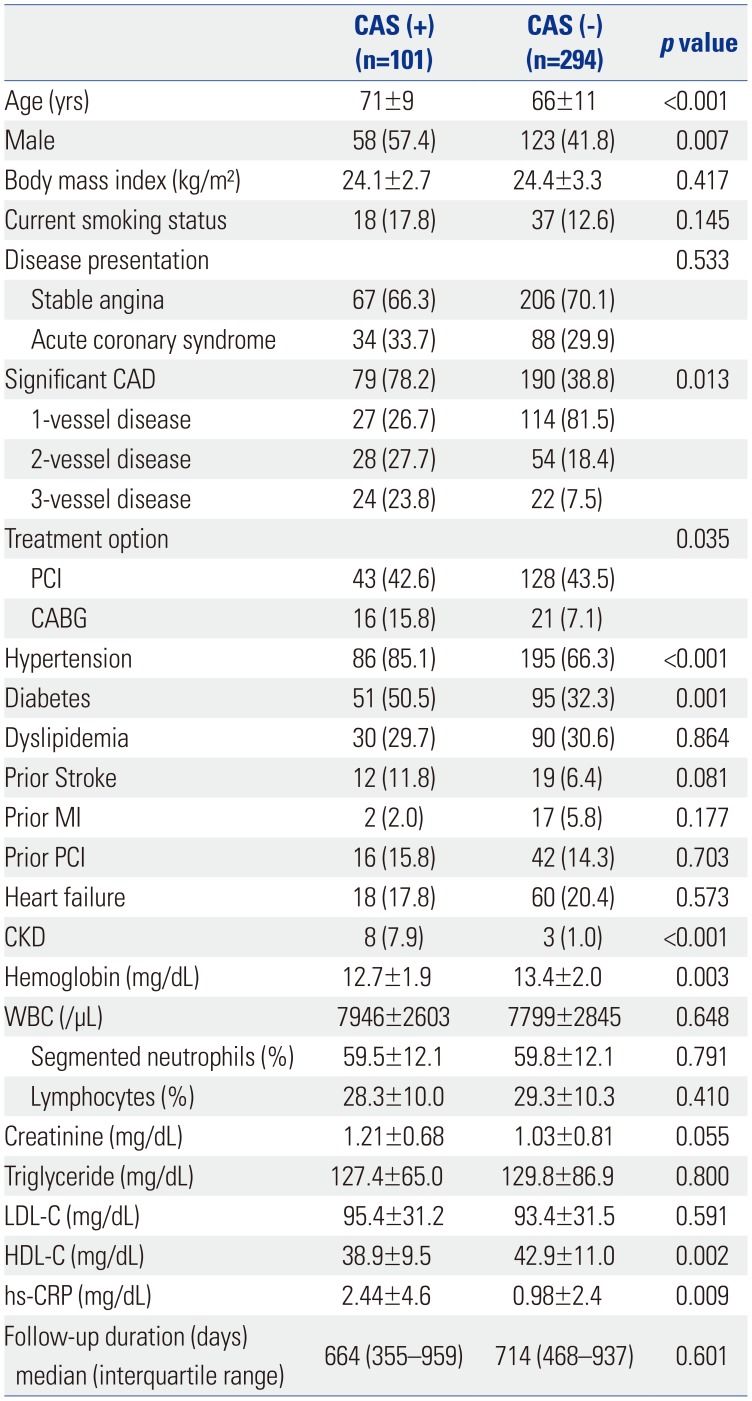
CAS, carotid artery stenosis; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CKD, chronic kidney disease; hs-CRP, high sensitivity C-reactive protein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; PCI, percutaneous coronary intervention; WBC, white blood cell.
Values are presented as a mean±standard deviation or numbers (percentage) unless otherwise noticed.
Table 2
Incidence of Baseline Coronary Artery and MACCE during Follow-Up
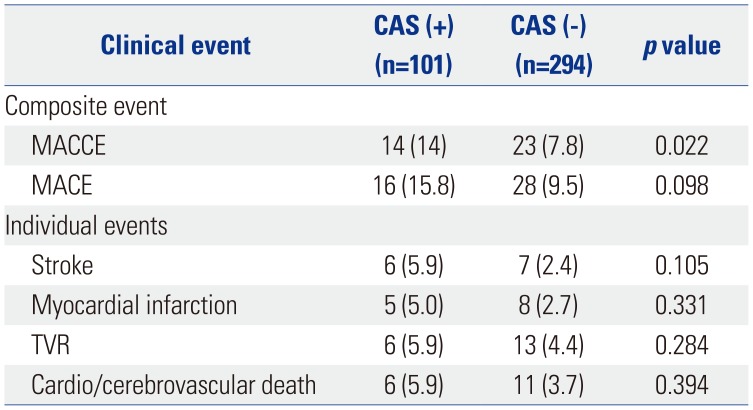
Table 3
Multivariate Analysis of the Prevalence of Carotid Artery Stenosis
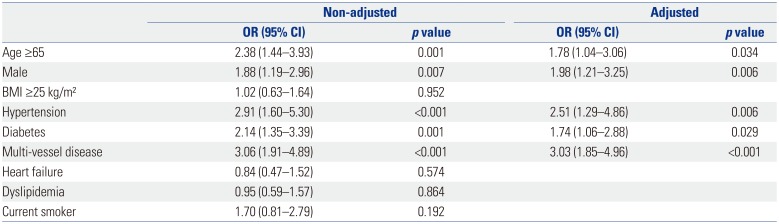
Table 4
Multivariate Analysis of the Prevalence of Major Adverse Cardiac and Cerebrovascular Event in Patients with Significant Coronary Artery Disease




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download