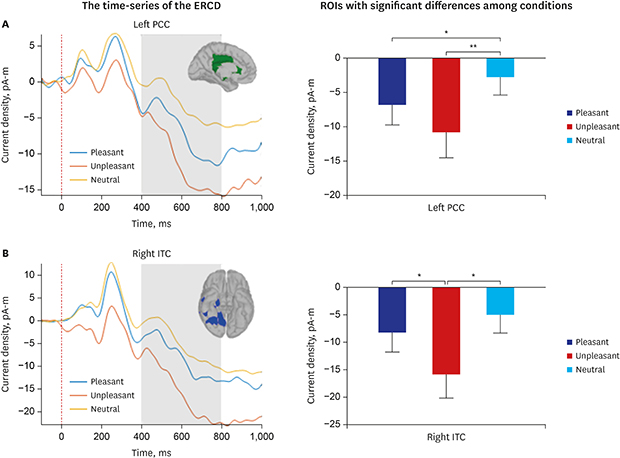
|
Pleasant (n = 100) |
1560, 1590, 1595, 1640, 1650, 1659, 1710, 1720, 1721, 1722, 1731, 1740, 1810, 1811, 1947, 1999, 2018, 2019, 2025, 2030, 2034, 2045, 2071, 2075, 2150, 2152, 2153, 2154, 2155, 2158, 2160, 2208, 2209, 2216, 2224, 2300, 2303, 2311, 2340, 2345, 2346, 2347, 2362, 2373, 2374, 2389, 2391, 2398, 2605, 2616, 4006, 4007, 4008, 4071, 4090, 4130, 4150, 4225, 4250, 4255, 4274, 4275, 4279, 4325, 4597, 4598, 4599, 4601, 4603, 4606, 4607, 4608, 4609, 4610, 4612, 4619, 4623, 4624, 4625, 4626, 4628, 4640, 4641, 4643, 4645, 4653, 5215, 5220, 5260, 5270, 5300, 5301, 5450, 5460, 5470, 5480, 5600, 5621, 5622, 8500 |
|
Unpleasant (n = 100) |
2095, 2301, 2717, 2190, 2800, 2811, 2900, 3000, 3001, 3010, 3015, 3016, 3017, 3030, 3051, 3053, 3059, 3060, 3061, 3062, 3063, 3064, 3068, 3069, 3071, 3080, 3100, 3101, 3102, 3103, 3110, 3120, 3130, 3131, 3140, 3150, 3160, 3168, 3170, 3180, 3191, 3195, 3215, 3220, 3225, 3230, 3261, 3266, 3301, 3350, 3400, 3500, 3530, 6021, 6022, 6212, 6230, 6231, 6243, 6250, 6260, 6311, 6312, 6313, 6315, 6350, 6360, 6415, 6510, 6520, 6530, 6540, 6560, 6563, 6570, 6821, 6831, 6838, 7380, 9000, 9006, 9007, 9040, 9075, 9140, 9163, 9180, 9181, 9183, 9184, 9185, 9187, 9250, 9252, 9253, 9254, 9265, 9280, 9920, 9911 |
|
Neutral (n = 200) |
1080, 1122, 2393, 5390, 7019, 7092, 7950, 2394, 5395, 7020, 7095, 8502, 1202, 2396, 5410, 7021, 7096, 8503, 1240, 2397, 5455, 7023, 7100, 8510, 1301, 2410, 5500, 7025, 7110, 8531, 1302, 2411, 5510, 7026, 7130, 8540, 1303, 2435, 5520, 7030, 7135, 8600, 1321, 2440, 5530, 7031, 7136, 8620, 1333, 2458, 5531, 7032, 7137, 1670, 2480, 5532, 7033, 7140, 1675, 2484, 5533, 7034, 7150, 1903, 2485, 5534, 7035, 7160, 1908, 2487, 5535, 7036, 7161, 1930, 2488, 5731, 7037, 7165, 1931, 2489, 5661, 7038, 7170, 1932, 2495, 5970, 7039, 7175, 1935, 2514, 5971, 7040, 7179, 2100, 2516, 5972, 7041, 7180, 2101, 2518, 6000, 7042, 7182, 2102, 2570, 6010, 7043, 7183, 2104, 2575, 6150, 7044, 7184, 2107, 2579, 6840, 7045, 7185, 2190, 2580, 6900, 7046, 7186, 2200, 2595, 6930, 7050, 7187, 2206, 2597, 6940, 7052, 7188, 2210, 2620, 7000, 7053, 7190, 2211, 2630, 7001, 7054, 7192, 2214, 2635, 7002, 7055, 7211, 2215, 2690, 7003, 7056, 7224, 2221, 2692, 7004, 7057, 7233, 2230, 2694, 7006, 7058, 7234, 2270, 2695, 7009, 7059, 7235, 2271, 2749, 7010, 7060, 7236, 2272, 2752, 7011, 7061, 7237, 2273, 2810, 7012, 7062, 7242, 2280, 2830, 7013, 7077, 7247, 2377, 2850, 7014, 7078, 7248, 2381, 2870, 7016, 7080, 7249, 2382, 3210 |









 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download