Abstract
Objective
Cancer metastasis is a complex process involving a sequential series of multi-step genetic events, which produces an imbalance between stimulatory and inhibitory genes for metastasis. Presently, we examined the expression of metastatic tumor antigen 1 (MTA1) and nonmetastatic protein 23 homologue H1 (nm23-H1) proteins in metastasized epithelial ovarian cancer cells.
Methods
Fifty-one primary epithelial ovarian tumors and corresponding lymph nodes (LNs) were examined immunohistochemically for expression of MTA1 and nm23-H1. Expression of these proteins was statistically evaluated.
Results
The frequency of MTA1 expression was 30.3% (10/33) in stage III/IV LNs but was absent (0/18) in stage I/II LNs (p=0.01). MTA1 expression was observed in 50% (6/12) of metastasizing LNs but in only 10.3% (4/39) of non-metastasizing LNs (p=0.01). In contrast with MTA1, nm23-H1 expression was evident in 16 of 18 (88.9%) stage I/II ovarian cancer tissue samples but only in 20 of 33 (60.6%) stage III/IV tissues (p=0.05), and nm23-H1 production was also observed in 75.6% (34/45) of ovarian cancer tissue with residual tumors under 2 cm in diameter, but in 2/6 (33.3%) of cancer tissue with residual tumors exceeding 2 cm in diameter (p=0.03).
According to statistics reported by the Cancer Committee of the Korean Obstetrics and Gynecology Association, advanced stage cancer is more common in patients with ovarian cancer at diagnosis than with cervical or endometrial cancer.1 Cancer-related morbidity and mortality are predominantly the result of tumor invasion and metastasis.2 Because distant metastasis is also one of the most important factors affecting prognosis in the patients with ovarian cancer, extensive efforts have been made to predict metastasis. However, metastasis is a complex process involving the expression of several genes including those important in the detachment of cancer cells from the primary tumor, penetration into the local vessels and lymphatics, arrest at distant sites by adhesion to endothelial cells and underlying matrix, extravasations, the induction of angiogenesis, escape from anti-tumor immune responses, and growth at metastatic sites.3
Metastatic tumor antigen 1 (MTA1) is the human homologue of the mta1 gene cloned by differential cDNA library screening using a rat carcinoma metastatic model.4-6 Expression of MTA1 It correlates with metastatic potential in several human cancers including gastric and colorectal carcinomas7 and esophageal cancer.8 Expression of the MTA1 gene is related to degree of invasion and lymphatic metastasis. On the contrary, the nonmetastatic protein 23 homologue H1 (nm23-H1) gene was initially cloned as a metastasis suppressor gene, with reduced nm23 mRNA levels correlating with higher metastatic potential in vitro.9 Transfection of human nm23-H1 cDNA into human MDA-MB-435 breast cancer cell lines significantly reduces the metastatic potential in vivo.10 In breast, gastric, and hepatocellular cancers, and in melamona, nm23 expression has been inversely correlated with metastatic potential and/or survival.11 However, high nm23 expression is associated with more aggressive disease progression in pancreatic cancer and neuroblastoma.12 Studies on ovarian cancer are few, and so the association between nm23 expression and prognosis is unclear and contentious.13-15
Here, we examined MTA1 and nm23-H1 expression in primary ovarian cancer and metastatic lymph nodes (LNs) to determine whether the expression of MTA1 and nm23-H1 are significant predictors of metastasis.
We studied 51 epithelial ovarian cancer samples that were over FIGO stage Ic obtained from 51 patients treated at Kyung-hee University Hospital between January 1993 and April 2004. Clinical and pathologic data were retrieved retrospectively from a database. The variables included patient age, disease stage, histologic type and differentiation, postoperative residual tumor size, LN status, and response to chemotherapeutic agents.
For MTA1 and nm23-H1 immunohistochemical staining, the CAP-Plus Detection Kit (Zymed Laboratories, San Francisco, CA) was used. Sections of 5 m thickness were cut from 10% formalin-fixed, paraffin-embedded tissue blocks. Deparaffinized sections were treated with methanol with 1.5% hydrogen peroxide (H2O2) for 20 min to block endogenous peroxidase. Cross reactivity was blocked with 2% nonimmune rabbit serum and 0.1% bovine serum albumin in phosphate buffered saline. Sections were then incubated with MTA1 specific monoclonal antibody A11 (1μg IgG1/ml) and nm23-H1 specific monoclonal antibody clone NM301 (5μg IgG1/ml) for 1 h at room temperature. The sections were then incubated with secondary anti-mouse IgG antibody and streptavidin-peroxidase for 20 min each. Tissue was stained for five min with 3'3'-diaminobenzidine. The primary cancer specimen and ovarian cancer LN tissue were used as positive controls for MTA1 and nm23-H1, respectively. For negative controls, sections were treated the same way, except they were incubated with buffered saline instead of the primary antibody. The intensity of cell staining was graded 1-4, corresponding to absence of stain, intensity <5%, intensity of 5-70%, and intensity >70%, respectively (Fig. 1, 2).
Statistical analyses were conducted using SPSS version 11.0 (SPSS, Chicago, IL). Relations between the expression of MTA1 and nm23-H1 and the clinicopathological parameters were evaluated via Chi-square tests. For all statistical tests, the level of significance was set at a p-value <0.05.
The median age at diagnosis of the 51 patients was 51 years (range 21-73 years). The pathological characteristics of the patients are shown in Table 1. There were 14 stage I cases (27.5%), 4 stage II cases (7.8%), 29 stage III cases (56.9%), and 4 stage IV cases (7.8%). Adenocarcinomas included serous (n=26, 51%), mucinous (n=14, 17.6%), and endometrioid (n=9, 17.6%). Forty-five (88.2%) patients had postoperative residual masses <2 cm in diameter and 6 patients (11.8%) had a residual mass ≥2 cm in diameter. Twelve (23.6%) cases were LN metastasis positive and 39 (76.4%) were LN metastasis negative. MTA1 expression was positive in 37 (72.5%) cases of primary cancer tissue and 10 (19.6%) cases of LN. Thirty-six (70.5%) cases showed positive staining for nm23-H1 in ovarian cancer tissue and 4 (7.8%) cases were LN positive. LNs for both MTA1 and nm23-H1 displayed expression that was below that of primary ovarian cancer tissue.
The relationship between the frequency of MTA1 expression and the clinicopathological parameters is shown in Table 2. For primary ovarian cancer tissue, MTA1 expression did not correlate with any of the clinicopathological parameters such as stage, histologic type and grade, residual tumor size, and lymph node metastasis. However, for LN staining, the absence of MTA1 expression in stage I/II LNs was significantly lower than the 30.3% level in stage III/IV LNs. MTA1 expression in metastasized LNs (50%) was significantly higher that non-metastasized LNs (10.3%).
The clinicopathological correlation between the expression level of the nm23-H1 protein and several parameters is shown in Table 3. In primary ovarian cancer tissue, the frequency of nm23-H1 expression in stage I/II (88.9%) was significantly higher than the frequency in stage III/IV (60.6%) (p=0.05). Patients whose residual tumor size was <2 cm (75.6%) were significantly more inclined to be nm23-H1 positive than patients with a residual tumor ≥2 cm (33.3%). No significant association was evident between nm23-H1 expression and the examined clinicopathological factors in LNs.
In the present study, we demonstrate that the expression of MTA1 in LNs is significantly associated with advanced stage (stage III/IV) and lymphatic metastasis. In contrast, expression of nm23-H1 is significantly associated with stage I/II tumor and small (<2 cm) residual tumor size.
MTA1 is normally expressed only at low levels in various tissues.5 However, in several human metastatic cancers, expression levels of MTA1 correlate with metastatic potential and degree of invasion.5,7,8,16-18 The relationship between MTA1 protein overexpression and the invasion/metastasis of cancer cells has also been also demonstrated in vitro. Increasing MTA1 expression enhances migration, invasion and anchorage-independent survival of immortalized human keratinocytes19 and in epithelial ovarian cancer cells.20 The latter study also showed that increased expression of MTA1 significantly correlates with the histological grade and stage of ovarian cancer. Our findings agree with these previous studies.
The nm23 gene is a metastasis-associated gene that maps to human chromosome band 17q22.21 The nm23 gene family consists of eight genes, of which nm23-H1 and H2 are the most studied members.22 These two genes encode nucleoside diphosphate kinases (NDPK) A and NDPK B, respectively. The nm23 protein seems to play an important, but as yet unclear, role in human cancer. Potential suggested roles for the nm23 protein include regulating cell signals by NDPK, GDP/GTP exchange,23 tumor differentiation,24 and cell proliferation.25
In epithelial ovarian cancer, high levels of nm23 expression have been linked to the increased metastatic spread of the cancer.13,14 In childhood neuroblastomas, mutations and/or genetic alterations of the nm23-H1 gene, together with overexpression associated with a more malignant phenotype, have also been reported.26 As well, association between nm23-H1 and the clinical significance of gastric cancer27 and breast cancer28 has been reported. However, our results demonstrate that the expression of nm23-H1 is decreased in advanced stage (III/IV) and when residual tumors are ≥2 cm in size. The present results, together with those of a previous study15 indicate that nm23-H1 overexpression might be a good prognostic factor for epithelial ovarian cancer. Indeed, nm23-H1 overexpression has been associated with both a survival advantage and greater response to chemotherapy in patients with advanced ovarian cancer, with the percentage of nm23-H1 positivity being higher in LN negative (70%) than in LN positive cases (40%), and the survival rate of nm23-H1 positive patients being higher than nm23-H1 negative patients.29
In conclusion, our results demonstrate the positive correlation between MTA1 expression and LN metastasis, and implicate nm23-H1 expression as a good prognostic factor in epithelial ovarian cancer. The expression of MTA1 and nm23-H1 might be of considerable importance as biologic predictive markers of LN metastasis of epithelial ovarian cancer. However, the exact mechanism of the pathogenesis of the two genes that gives rise to the metastasis is unknown and in need of further study.
Figures and Tables
 | Fig. 1Representative images of tissue microarray samples staining absent, weak, moderate, and strong for MTA1 expression. MTA1 expression was confined to the nuclei of cells as demonstrated by immunohistochemistry (×200 magnification). |
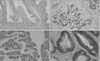 | Fig. 2Representative images of tissue microarray samples staining absent, weak, moderate, and strong for nm23-H1 expression. nm23-H1 expression was confined to the nuclei of cells as demonstrated by immunohistochemistry (×200 magnification). |
References
1. Annual report of gynecologic cancer registry program in Korea for 2004 (Jan. 1st, 2004-Dec, 31st, 2004). Korean J Obstet Gynecol. 2007. 50:28–78.
2. Fidler IJ. Critical factors in the biology of human cancer metastasis: Twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990. 50:6130–6138.

3. Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: An imbalance of positive and negative regulation. Cell. 1991. 64:327–336.

4. Pencil SD, Toh Y, Nicolson GL. Candidate metastasis-associated genes of the rat 13762NF mammary adenocarcinoma. Breast Cancer Res Treat. 1993. 25:165–174.
5. Toh Y, Pencil SD, Nicolson GL. A novel candidate metastasis-associated gene, mta1, differentially expressed in highly metastatic mammary adenocarcinoma cell lines. cDNA cloning, expression, and protein analyses. J Biol Chem. 1994. 269:22958–22963.
6. Toh Y, Pencil SD, Nicolson GL. Analysis of the complete sequence of the novel metastasis-associated candidate gene, mta1, differentially expressed in mammary adenocarcinoma and breast cancer cell lines. Gene. 1995. 159:97–104.

7. Toh Y, Oki E, Oda S, Tokunaga E, Ohno S, Maehara Y, et al. Overexpression of the MTA1 gene in gastrointestinal carcinomas: Correlation with invasion and metastasis. Int J Cancer. 1997. 74:459–463.

8. Toh Y, Kuwano H, Mori M, Nicolson GL, Sugimachi K. Overexpression of metastasis-associated MTA1 mRNA in invasive oesophageal carcinomas. Br J Cancer. 1999. 79:1723–1726.

9. Steeg PS, Bevilacqua G, Kopper L, Thorgeirsson UP, Talmadge JE, Liotta LA, et al. Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst. 1988. 80:200–204.

10. Leone A, Flatow U, VanHoutte K, Steeg PS. Transfection of human nm23-H1 into the human MDA-MB-435 breast carcinoma cell line: Effects on tumor metastatic potential, colonization and enzymatic activity. Oncogene. 1993. 8:2325–2333.
11. de la Rosa A, Williams RL, Steeg PS. Nm23/nucleoside diphosphate kinase: Toward a structural and biochemical understanding of its biological functions. Bioessays. 1995. 17:53–62.
12. Leone A, Seeger RC, Hong CM, Hu YY, Arboleda MJ, Brodeur GM, et al. Evidence for nm23 RNA overexpression, DNA amplification and mutation in aggressive childhood neuroblastomas. Oncogene. 1993. 8:855–865.
13. Srivatsa PJ, Cliby WA, Keeney GL, Dodson MK, Suman VJ, Roche PC, et al. Elevated nm23 protein expression is correlated with diminished progressionfree survival in patients with epithelial ovarian carcinoma. Gynecol Oncol. 1996. 60:363–372.
14. Leary JA, Kerr J, Chenevix-Trench G, Doris CP, Hurst T, Houghton CR, et al. Increased expression of the NME1 gene is associated with metastasis in epithelial ovarian cancer. Int J Cancer. 1995. 64:189–195.
15. Ferrandina G, Scambia G, Marone M, Benedetti Panici P, Giannitelli C, Pernisco S, et al. nm23 in ovarian cancer. Correlation with clinicopathological and biochemical parameters. Ann N Y Acad Sci. 1996. 784:509–512.
16. Iguchi H, Imura G, Toh Y, Ogata Y. Expression of MTA1, a metastasis-associated gene with histone deacetylase activity in pancreatic cancer. Int J Oncol. 2000. 16:1211–1241.

17. Sasaki H, Moriyama S, Nakashima Y, Kobayashi Y, Yukiue H, Kaji M, et al. Expression of the MTA1 mRNA in advanced lung cacner. Lung Cancer. 2002. 35:149–154.

18. Sasaki H, Yukiue H, Kobayashi Y, Nakashima Y, Kaji M, Fukai I, et al. Expression of the MTA1 mRNA in thymoma patients. Cancer Lett. 2001. 174:159–163.
19. Mahoney MG, Simpsom A, Jost M, Noe M, Kari C, Pepe D, et al. Metastasis-associated protein (MTA1) enhances migration, invasion, and anchorageindependent survival of immortalized human keratinocytes. Oncogene. 2002. 21:2161–2170.
20. Yoon MS, Jang SK, Lee DH, Kim KH, Na YJ, Kim JY, et al. MTA1 expression in epithelial ovarian neoplasm. Korean J Obstet Gynecol. 2006. 49:1463–1470.

21. Varesco L, Caligo MA, Simi P, Black DM, Nardini V, Casarino L, et al. The NM23 gene maps to human chromosome band 17q22 and shows a restriction fragment length polymorphism with BglII. Genes Chromosomes Cancer. 1992. 4:84–88.

22. Lacomb ML, Milon L, Munier A, Mehus JG, Lambeth DO. The human nm23/nucleoside diphosphate kinases. J Bioenerg Biomembr. 2000. 32:247–258.
23. Zhu J, Tseng YH, Kantor JD, Rhodes CJ, Zetter BR, Moyers JS, et al. Interaction of the Ras-related protein associated with diabetes rad and the putative tumor metastasis suppressor NM23 provides a novel mechanism of GTPase regulation. Proc Natl Acad Sci USA. 1999. 96:14911–14918.
24. Lombardi D, Sacchi A, D'Angostino G, Tibursi G. The association of the Nm23-H1 protein and beta-tubulin correlates with cell differentiation. Exp Cell Res. 1995. 217:267–271.
25. Caligo MA, Cipollini G, Fiore L, Calvo S, Basolo F, Collecchi P, et al. NM23 gene expression correlates with cell growth rate and S-phase. Int J Cancer. 1995. 60:837–842.
26. Leone A, Flatow U, Van Houtte K, Steeg PS. Transfection of human nm23H1 into human MDAMB-435 breast carcinoma cell line: Effects on tumour metastatic potential, colonization and enzymatic activity. Oncogene. 1993. 8:2325–2333.
27. Hwang SH, Oh SH, Choi YK. The clinical significance of mutation in the p53, DCC and nm23 genes in patients with gastric carcinoma. Korean J Gastroenterol. 2001. 38:325–335.
28. Bae JW, Kim J, Cho MY, Lee ES, Koo BH. Nm23 protein as a prognostic factor in lymph node negative breast cancers. J Korean Surg Soc. 1999. 57:836–842.
29. Scambia G, Ferrandina G, Marone M, Benedetti Panici P, Giannitelli C, Piantelli M, et al. Nm23 in ovarian cancer: Correlation with clinical outcome and other clinico-pathological and biochemical prognostic parameters. J Clin Oncol. 1996. 14:334–342.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download