Abstract
Objective
The conventional Papanicolaou smear seems to be more accurate for detecting a high-grade squamous intraepithelial lesion (HSIL) than a low-grade squamous intraepithelial lesion (LSIL). The purpose of this study was to investigate false-negative results of conventional Pap smear cytology in women with cervical conization.
Methods
This study was performed in Gynecologic Oncology Clinic of the Department of Obstetrics and Gynecology at Dankook University Medical Center from July 1, 1994 to December 31, 2004. 260 women from age 22 to 75 years had undergone conventional Papanicolaou cervical cytologic test and cervical conization. Conization was performed using 'cold-knife cone' method or 'large electrosurgical excision procedure (LEEP) cone' method. The cervical cytology were studied in comparison with histology of conization specimens.
Results
Of the 260 histologic diagnoses, there were 41 (15.8%) diagnoses of chronic cervicitis, 8 (3.1%) of CIN 1, 18 (6.9%) of CIN 2, 150 (57.7%) of CIN 3, 38 (14.6%) of SCC, 2 (0.8%) of AIS, 1 (0.4%) of ACC, and 2 (0.8%) of ASC. Pap cytology showed sensitivity of 97.9-98.2%, specificity of 4.9-6.7%, and false-negative rate of 1.8-2.2% according to the variables.
Cancer of the cervix uteri is the second most common cancer among women worldwide, with an estimated 493,000 new cases and 274,000 deaths in 2002.1 Some 83% of the cases occur in developing countries, where cervical cancer accounts for 15% of female cancers, with a risk before age 65 of 1.5%, while in developed countries it accounts for only 3.6% of new cancers, with a cumulative risk (ages 0-64) of 0.8%.1 The highest incidence rates are observed in sub-Saharan Africa, Melanesia, Latin America and the Caribbean, South Central Asia, and South East Asia.2
Mortality rates are substantially lower than incidence. Worldwide, the ratio of mortality to incidence is 55%. Survival rates vary between regions. Because of cervical cancer affects relatively young women, it is an important cause of lost years of life in the developing world.2 Yang et al.3 found that it was responsible for 2.7 million (age-weighted) years of life lost worldwide in 2000 and it is the biggest single cause of years of life lost from cancer in developing world. But uniquely, cervical cancer remains eminently preventable. The key to prevention is the timely identification and management of precancerous lesions through accessible and affordable screening programs.4
Traditional cervical screening methods are conventional Papanicolaou smear cytology and the pelvic examination. Since Dr. George Papanicolaou introduced this test for cervical cancer in 1939, the mortality rate for cervical squamous cell carcinoma in the United States decreased by 70-75% between 1955 and 1992. Today over 50 million tests are conducted annually in the United States.5 But unfortunately, this method contains numerous inherent opportunities for error leading to an acceptably high false negative rate. Many researchers have reported a false negative rate from 6% to 55%.6-10 The issue of false negative Pap smears was drawn to public attention in 1987 following a series of invetigative reports in the Wall Street Journal (November 2, 1987:1, 20; December 29, 1987: 17). Linder and Zahniser11 summarized that the consequences of this and subsequent reports include the implementation of the Clinical Laboratory Improvement Amendments of 1988,12 an erosion in Public confidence in the Pap smear, an unprecedented liability crisis for those who practice cervical cytology,13 a dramatic increase in the percentage of cases designated ;atypical squamous cells of undetermined significance (ASCUS); as cytopathologists attempt to protect themselves from potential liability,14 overuse of colposcopy and cervical biopsy in treating women with ASCUS Pap,15 and the application of new technology to Pap testing. Researchers have shown that false negative errors-53-90% of total false negative errors are due to sampling and preparation rather than interpretation errors.8,10
In February 1999, a report from AHCPR (the Agency for Health Care Policy and Research) in the US admitted that estimates of the sensitivity of conventional Pap screening are not as high as previously reported.16 The report mentioned that, based on the few studies that avoided severe biases, conventional Pap smear cytology showed sensitivity of 51% and specificity of 98%. Furthermore, the report stated that the conventional Pap test is more accurate when an high-grade squamous intraepithelial lesion (HSIL) threshold is used, with the goal of detecting a high-grade lesion, than when lower thresholds, such as a low-grade squamous intraepithelial lesion (LSIL) or ASCUS, are used, with the goal of detecting low or high-grade dysplasia.
Conization of the cervix is both a diagnostic and therapeutic procedure and has the advantage over ablative therapies of providing tissue for further evaluation to rule out invasive cancer. Conization is indicated for diagnosis in women with HSIL based on a Pap test under the various conditions.17 The purpose of this study was to investigate false-negative results of conventional Pap smear cytology in women with cervical conization in order to estimate diagnostic efficacy of conventional Pap smear cytology for detecting a high-grade lesion of the cervix.
The study design was a retrospective, university hospital-based design in which the false-negative results of conventional Pap smear cytology were to be evaluated in women who received cervical conization. This study was performed in the Gynecologic Oncology Clinic of the Department of Obstetrics and Gynecology at Dankook University Medical Center from July 1, 1994 to December 31, 2004. Conventional Pap smear test was compared with a reference standard, the histologic diagnosis by conization biopsy. The main outcome measures are the rate of correspondence, sensitivity, specificity, and false-negative rate of Pap smear cytology relative to the histologic diagnosis.
260 women had undergone conventional Papanicolaou cervical cytologic test and cervical conization. Conization was indicated under the following conditions: 1) Limits of the lesion could not be visualized with colposcopy, 2) The squamocolumnar junction (SCJ) was not seen at colposcopy, 3) There was a lack of correlation between cytology, biopsy, and colposcopy results.
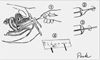
The cervical sample was taken in the usual way by the physician using a combination of plastic spatula and endocervical brush (cytobrush). Following collection according to the guidelines, the collection device was spread on the surface of the slide as evenly and thinly as possible (Fig. 1).18 Immediately the slide was fixed in 95% ethyl alcohol solution. The slide and a case report form containing the patient's identification number, initials and medical history were sent to the pathology laboratory.
The slides were prepared with the laboratory's routine Papanicolaou staining method, and evaluated according to The Bethesda System (TBS 1991, 1994, 2001) criteria. In brief, specimens that are not within normal limits can contain changes deemed either as benign cellular changes, or as cells that are consistent with an epithelial cell abnormality. The latter category includes precancerous cells that are consistent with low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL) or squamous cell carcinoma (SCC), or cells that are atypical but cannot be categorized as intraepithelial lesion. These atypical cells are usually referred to by their acronyms, ASCUS (atypical squamous cells of undetermined significance) and AGUS (atypical glandular cells of undetermined significance). Pap slides were initially screened by cytotechnologists, and finally confirmed by a pathologist. False-negative cases were re-interpreted by a pathologist.
Conization was performed using 'cold-knife cone' method or 'large electrosurgical excision procedure (LEEP) cone' method after Schiller test at operating room. The biopsy specimen was fixed in 10% buffered formalin, paraffin embedded, and prepared as conventional histopathologic slides stained with hematoxylin and eosin. The biopsies were interpreted and diagnosed using a combined reporting system, including TBS and CIN (cervical intraepithelial neoplasia) designations by a pathologist.
The 260 women had undergone conventional Papanicolaou cervical cytologic test and cervical conization. The mean age was 43.9 years (ranging from 22 to 75 years). The mean gravidity was 4.3 (from 0 to 18) and the mean parity was 2.5 (from 0 to 10). The mean number of abortion was 1.8 (from 0 to 12) and the mean number of living child was 2.4 (from 0 to 9).
On histology of the 260 cervical conization specimens, there were 41 (15.8%) cases of chronic cervicitis, 8 (3.1%) of CIN 1, 18 (6.9%) of CIN 2, 150 (57.7%) of CIN 3, 38 (14.6%) of SCC, 2 (0.8%) of AIS, 1 (0.4%) of ACC, and 2 (0.8%) of ASC in the histologic series.
On Pap cytology, there were 6 (2.3%) cases of negative, 40 (15.4%) of ASCUS, 24 (9.2%) of LSIL, 158 (60.8%) of HSIL, 29 (11.2%) of SCC, and 3 (1.2%) of AGUS (Table 1). Table 1 showed correlation between cervical cytologic diagnoses and histologic diagnoses. Thirty eight histologic diagnoses of SCC included 1 of negative, 4 of ASCUS, 20 of HSIL, and 13 of SCC, in the cytologic diagnosis. Eight histologic diagnoses of CIN 1 included 1 of negative, 2 of ASCUS, 2 of LSIL, 2 of HSIL, and 1 of AGUS, in the cytologic diagnosis. When ASCUS and AGUS were excluded, Pap smear cytology showed 2 true-negative, 4 false-negative, 28 false-positive, and 183 true-positive, in the histology comparison study. Diagnostic accuracy of Pap smear cytology showed sensitivity of 97.9% (183/187), specificity of 6.7% (2/30), positive predictive value of 86.7% (183/211), negative predictive value of 33.3% (2/6), false positive rate of 93.3% (28/30), and false-negative rate of 2.1% (4/187). When ASCUS and AGUS were included, Pap smear cytology showed 2 true-negative, 4 false-negative, 39 false-positive, and 215 true-positive, in the histology comparison study. Diagnostic accuracy of Pap smear cytology showed sensitivity of 98.2% (215/219), specificity of 4.9% (2/41), positive predictive value of 84.6% (215/254), negative predictive value of 33.3% (2/6), false positive rate of 95.1% (39/41), and false-negative rate of 1.8% (4/219).
Table 2 showed false-negative results of cervical cytologic diagnoses especially in squamous cell abnormal lesions of the cervix. When ASCUS was included and benign lesion or glandular lesions (AGUS, AIS, ACC, and ASC) were excluded, there were 4 cases of false-negative diagnosis. False-negative rate showed 14.3% in CIN 1, 5.6% in CIN 2, 0.7% in CIN 3, and 2.6% in SCC. Overall false-negative rate was 1.9%. The cause of 4 false-negative cases revealed sampling errors. When ASCUS and benign lesion or glandular lesions (AGUS, AIS, ACC, and ASC) were excluded, there were 4 cases of false-negative diagnosis. False-negative rate showed 20.0% in CIN 1, 5.9% in CIN 2, 0.8% in CIN 3, and 2.9% in SCC. Overall false-negative rate was 2.2%.
Conization of the cervix plays an important role in the management of CIN. Before the availability of colposcopy, conization was the standard method of evaluating an abnormal Pap test result. Conization is both a diagnostic and therapeutic procedure and has the advantage over ablative therapies of providing tissue for further evaluation to rule out invasive cervical cancer. Conization is indicated for diagnosis in women with HSIL based on a Pap test under the various conditions.17
The data of this study are consistent with results of other studies.16,19,20 Pairwuiti19 reported that false-negative rate showed 2.8% in CIS and 1.2% in SCC. Kim, et al20 reported that false-negative rate showed 12.3% in LSIL, 4.8% in HSIL, and 2.1% in SCC. A report from AHCPR16 showed the metaanalysis data from the 84 studies of conventional Pap tests. It reported that the summary effectiveness scores ranged from 1.03 (95% confidence interval [CI]; 0.78 to 1.14) for ASCUS/CIN 1 to 1.29 (95% CI; 1.08 to 1.50) for HSIL/CIN 2-3. Also the report mentioned that although the effectiveness score for each threshold was relatively low, better discrimination was seen with higher cytological and histological threshold.
Traditional cervical screening methods are pelvic examination and conventional Pap smear cytology. However, Pap smear cytology alone has the limitations of relatively low sensitivity and a high false negative rate. In February 1999, a report from AHCPR in the US admitted that estimates of the sensitivity of conventional Pap screening are not as high as previously reported.16 Inherent drawbacks in Pap smear cytology can lead to inaccurate diagnoses of cervical lesions. Pap smear cytology is completely reliant upon exfoliated cell samples. Errors may occur in the process of sampling, preparation, and interpretation. Researchers have shown that sampling and preparation errors form the major contribution to the high false negative rate. While an average sample obtained during a Pap smear test will contain between 300,000 to 500,000 cells, abnormal cells may not be present on the slide in any case of the following: 1) the lesions don't exfoliate abnormal cells, 2) the exfoliated cells are trapped beneath a barrier, 3) the lesions exist in tissue below the surface where they are unavailable for exfoliation, 4) the abnormal cells may simply be missed by the collecting device, or 5) the collecting device retrieves an accurate sampling but it may transfer only normal cells to the slide.21 Some researchers reported that more than 80% of the cell sample is thrown away with the sampling device into the trash at the doctor's office, and that the remaining sample on the slide may not necessarily represent the condition of the cervix. Sherman et al22 reported that intensive research re-screening studies, in contract, have suggested that as many as 50-90% of false negatives may be due to the limitations of vigilance and recognition in conventional screening and that computer assisted screening can further minimize this source of error.
The ThinPrep Pap Test (Cytyc Corporation, Boxborough, MA) is a liquid-based cell collection method that was approved by the United States Food and Drug Administration (FDA) as a replacement for the conventional Pap smear test for cervical cancer screening in May, 1996.
In August 1998, The American College of Obstetricians and Gynecologists Committee on Gynecologic Practice issued a committee opinion, "New Pap Test screening Techniques." The report concludes, "These new techniques improve the sensitivity of cervical cytology and reduce, but do not eliminate false negative Pap test." In February 1999, a report from AHCPR16 admits that estimates of sensitivity of conventional Pap screening are not as high as previously reported. They also mentioned that few studies of the new technologies used histology or colposcopy as a reference standard or allowed estimates of both sensitivity and specificity. They state that "Although it is clear that both thin-layer cytology and computerized re-screening technologies provide an improvement in effectiveness at higher cost, the imprecision in estimates of effectiveness makes drawing conclusions about the relative cost-effectiveness of thin-layer cytology and computerized re-screening technologies problematic." In March 2000, The International Consensus Conference on the Fight Against Cervical Cancer, IAC Task Force 3 Summary24 concludes that there have been no well-controlled, randomized, prospective clinical trials comparing liquid-based systems with conventional cervical cytology, and until this evidence is available, the conventional Pap smear should remain the international standard of care for the diagnosis of cervical cancer precursors in cancer screening programs. In July 2003, Abulafia, et al25 mentioned that ThinPrep cytology was reported as normal in 93.5% of cases of normal conventional Pap smears in the evaluation study of 24 English-language articles. They reported that the remaining 6.5% of ThinPrep slides were classified as follows: atypical, 4.55%; LSIL, 1.56%; HSIL, 0.36%; invasive cancer, 0.007%.
Squamous cancer of the cervix accounts for approximately 80-85% of invasive cervical cancer cases. Adenocarcinoma, which accounts for another 10-15%, may be increasing in incidence. Cervical cytology may also be less sensitive for adenocarcinoma. Therefore cervical cancer prevention requires eradication of pre-cancerous lesion known as CIN. Liquid-based cytology seems to be superior to conventional Pap cytology because of sample adequacy for detecting pre-cancerous lesions of the cervix. The central purpose of cervical cancer screening is the detection and management of high-grade lesions, particularly CIN 3 (severe dysplasia and carcinoma in situ). The data in this study revealed that false-negative rate of the squamous cell abnormalities showed 5.6% (1/18) in CIN 2, 0.7% (1/150) in CIN 3, and 2.6% (1/38) in SCC. Overall false-negative rate in high-grade lesions (CIN 2 or worse) showed 1.5% (3/206) (Table 2). The results of this study indicate that false-negative rate of Pap cytology in women with conization was significantly very low. And so, these data are thought to confirm the accuracy of the conventional Pap cytology for diagnosing high-grade cervical lesions as well as liquid-based cytology.
Because of inherent drawbacks in Pap smear cytology, many kinds of ancillary or adjunctive tests including liquid-based cytology for cervical cancer screening were developed in order to increase the sensitivity and decrease the false-negative rate4,18 In-vivo adjunctive tests assess the cervix directly. Visual tests such as colposcopy, speculoscopy, and cervicography evaluate the cervix for the presence of identifiable abnormalities, and are not dependent on exfoliation. In these tests diseased areas of the cervix, including areas of dysplasia, have larger nuclei than normal cervical cells. As a result, they have a higher N/C (nuclear:cytoplasmic) ratio. These cells will not allow light to pass through as well as normal cells do, and the underlying vascular layer is not seen. Thus the absence of color or whitening is seen, while the normal tissue appears pink. Pap smear cytology and visual tests are sensitive to different properties of cervical lesions, and so these two tests are theoretically additive from the viewpoint of cellular pathophysiology.21 Soler and Blumenthal4 reported three current trends toward improving cervical cancer screening: the first is to improve the test qualities of cytology-based screening (liquid-based cytology and computerized analysis of Papanicolaou test), the second is to improve the test qualities through various combinations of parallel or sequential tests, and the third is the possibility to make use of advances in digital and spectroscopic techniques.4,18
The data from this study demonstrate that false-negative rate of Pap cytology in women with conization was very low. And so, conventional Pap cytology is still clinically efficient for screening high-grade cervical lesions with adequate sampling technique.
Figures and Tables
Fig. 1
Preferred procedure for obtaining a Papanicolaou smear. ➀ Expose the entire cervix including transformation zone through the speculum, as much as possible. ➁ Rotate Ayre type spatula 360℃ twice. ➂ Rotate Endocervical cytobrush 180-360℃ softly. ➃ Spread cells in spatula on the upper half area of the slide, and roll brush over the lower half area of the same slide.
ACKNOWLEDGEMENT
I would like thank Yun-Dan Kang, assistant professor in the Department of Obstetrics and Gynecology at Dankook University Medical Center for data collection, computer work, and statistical analysis.
References
1. Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002 cancer incidence Mortality and prevalence worldwide. IARC CancerBase No. 5 Version 2.0. 2004. Lyon: IARC Press.
2. Parkin DM, Bray F. The burden of HPV-related cancers. Vaccine. 2006. 24:S311–25.
3. Yang BH, Bray FI, Parkin DM, Sellors JW, Zhang ZF. Cervical cancer as a priority for prevention in different world regions: an evaluation using years of life lost. Int J Cancer. 2004. 109:418–424.

4. Soler ME, Blumenthal PD. New technologies in cervical cancer precursor detection. Curr Opin Oncol. 2000. 12:460–465.

5. Grohs DH. Challenges in cervical cancer screening: what clinicians, patients and the general public need to know. Acta Cytol. 1996. 40:133–137.
6. Richart RM, Valiant HW. Influence of cell collection techniques upon cervical diagnosis. Cancer. 1965. 18:1474–1478.
7. Coppleson LW, Brown B. Estimation of the screening error rate from the observed detection rates in reported cervical cytology. Am J Obstet Gynecol. 1974. 119:953–958.
8. Gay JD, Donaldson LD, Goellner JR. False-negative rate in cervical cytologic studies. Acta Cytol. 1985. 29:1043–1046.
9. Koss LG. The Papnicolaou test for cervical cancer detection: A triumph and a tragedy. J Am Med Assoc. 1989. 261:737–743.
11. Linder J, Zahniser D. ThinPrep Papanicolaou testing to reduce false-negative cervical cytology. Arch Pathol Lab Med. 1998. 122:139–144.
12. Health Care Financing Administration. Medicare, Medicaid and CLIA programs; regulations implementing the Clinical Laboratory Improvement Amendments of 1988: proposed rule. Federal Register. 1990. May. 21. 55:10908.
13. Frable WJ. Litigation cells: definition and observations on a cell type in cervical vaginal smears not addressed by the Bethesda System. Diagn Cytopathol. 1994. 11:213–215.

16. AHCPR(Agency for Health Care Policy and Research). Evaluation of cervical cytology. Evidence report/Technology assessment. No. 5. 1999.
17. Berek JS. Novak's Gynecology. 2002. 13th ed. Philadelphia: Lippincott Williams & Wilkins;491.
18. Park CH. Cervical Cytology. 2004. 1st ed. Cheonan: Hanyoung;61–96.
19. Pairwuiti S. False negative Papanicolaou smears from woman with cancerous and precancerous lesions of the uterine cervix. Acta Cytol. 1991. 35:40–46.
20. Kim HS, Back HS, Son CW, Chung HW, Lee KH, Shim JU, et al. False-negative cytology in cervical smears-An evaluation on 1,000 cases of suamous intraepithelial lesion and squamous cell carcinoma histologically confirmed. Korean J Gynecol Oncol Colpo. 1995. 6:31–37.
21. Trylon Corporation. PapSure®: Background information and technology description. Accessed November 2000. Available at:
http://www.papsure.com/what.asp.
22. Sherman ME, Mango LJ, Kelly D, Paull G, Ludin V, Copeland C, et al. PAPNET analysis of reportedly negative smears preceding the diagnosis of a high-grade squamous intraepithelial lesion or carcinoma. Mod Pathol. 1994. 7:578–581.
23. Linder J, Zahniser D. The ThinPrep Pap Test: A review of clinical studies. Acta Cytol. 1997. 41:30–38.
24. The International Consensus Conference on the Fight Against Cervical Cancer, IAC Task Force 3 Summary, Chicago, Illinois, USA. In March 2000. Sampling, Sampling Errors and Specimen preparation. Acta Cytol. 2000. 44:944–948.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download