This article has been
cited by other articles in ScienceCentral.
Abstract
Background
We investigated the clinical outcome in patients whose cavitary bone defects were treated with beta-tricalcium phosphate (β-TCP) after surgical removal of benign tumors.
Methods
Between March 2015 and December 2015, 20 patients who underwent operation for bone tumors were enrolled into this study and prospectively followed up for a median period of 28.1 months.
Results
When the radiographic sign of complete resorption was defined as greater than 50% resorption of the allograft material accompanied by bone remodeling until 12 months, 55% of patients had complete resorption. Positive correlation between the filling volume and time needed for complete resorption was not found (p = 0.184).
Conclusions
Purified β-TCP could be a suitable choice as a bone graft substitute after the removal of benign bone tumors.
Go to :

Keywords: Beta-tricalcium phosphate, Bone transplantation
Autogenous bone grafting has been widely used to fill the defects caused by fracture, trauma, tumor resection, or arthrodesis. However, it has been reported to cause complications such as infection, hematoma, nerve injury, donor defect herniation, and fracture at the harvest site.
123) To avoid these complications, the use of bone graft substitutes has been gradually increased.
45) Beta-tricalcium phosphate (β-TCP) is one of those substitutes whose use has dramatically increased in recent years.
678) It is one of the first calcium phosphate compounds that was started to be used as a synthetic bone graft substitute.
910) This bioactive ceramic was known to be safe, nontoxic, safe from disease transmission, and free from immunogenic response. We prospectively analyzed the clinical outcome of patients treated with β-TCP to determine the adequacy of β-TCP as a bone graft substitute after benign bone tumor surgery.
METHODS
We conducted this study in compliance with the principles of the Declaration of Helsinki. The design and protocol of this prospective study were approved by the Institutional Review Board of Kosin University Gospel Hospital (IRB No. 2015-02-102), and written informed consents were obtained from all patients.
Between March 2015 and December 2015, 20 patients scheduled for surgical treatment of benign bone tumors were enrolled into the prospective study. β-TCP (ExcelOS; CGBio Inc., Seongnam, Korea) was used in these patients. Patients were excluded from the study if they had a malignant bone tumor or surgical wound infection and history of reoperation or pregnancy.
The median age of the 20 patients was 38.5 years. Twelve of the patients were male and eight were female (
Table 1). The anatomical distributions and types of tumors are summarized in
Table 1. After complete curettage of the tumor, the bone defect was filled with β-TCP granules. β-TCP (Ca3(PO4)2, ExcelOS; CGBio Inc.) used in this study is macroporous with a total pore volume of 45% ± 5% and a pore size between 250 and 400 µm. All patients were followed up for minimum 2 years. The median period of follow-up was 28.1 months (range, 24.1 to 38.4 months) (
Table 1).
Table 1
Patient Demographic Data
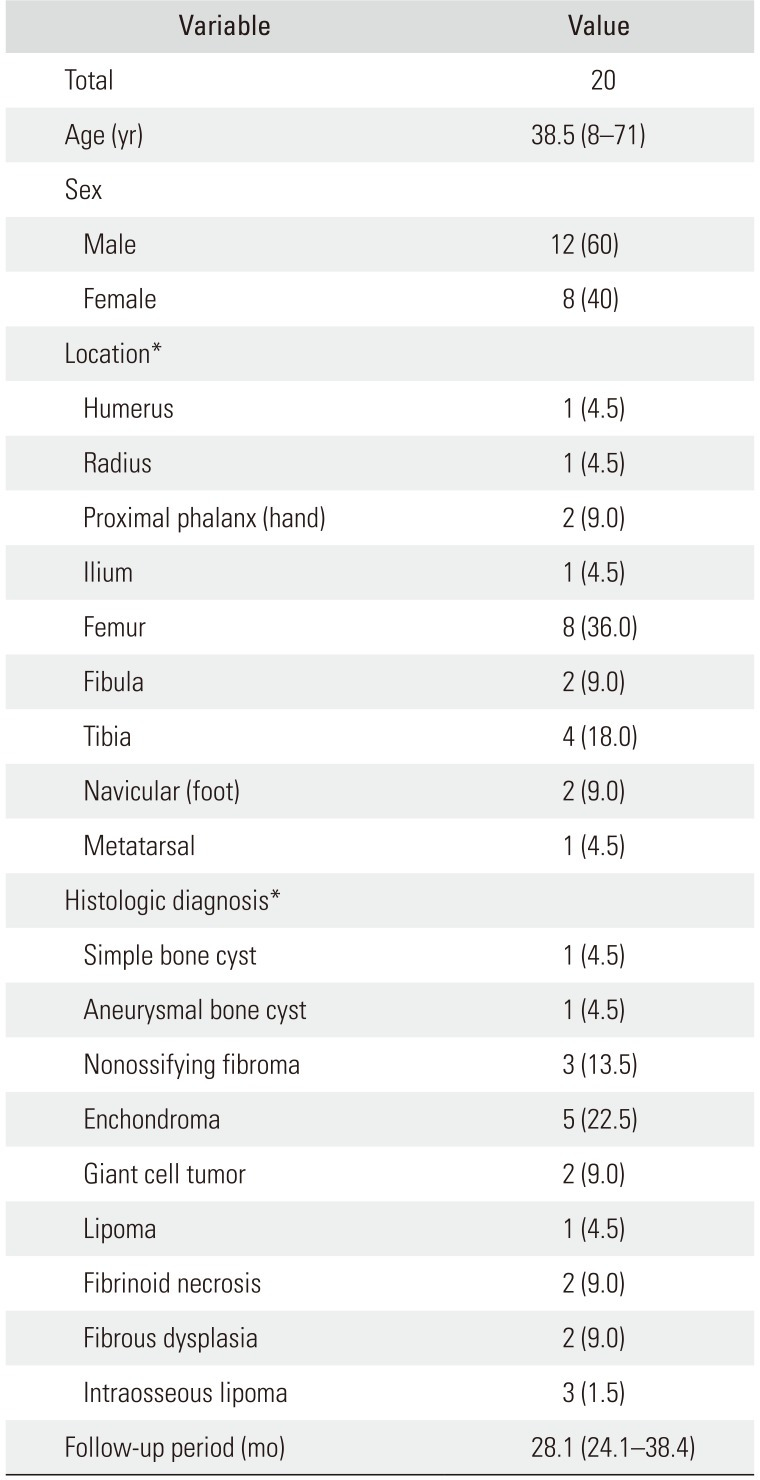
|
Variable |
Value |
|
Total |
20 |
|
Age (yr) |
38.5 (8–71) |
|
Sex |
|
|
Male |
12 (60) |
|
Female |
8 (40) |
|
Location*
|
|
|
Humerus |
1 (4.5) |
|
Radius |
1 (4.5) |
|
Proximal phalanx (hand) |
2 (9.0) |
|
Ilium |
1 (4.5) |
|
Femur |
8 (36.0) |
|
Fibula |
2 (9.0) |
|
Tibia |
4 (18.0) |
|
Navicular (foot) |
2 (9.0) |
|
Metatarsal |
1 (4.5) |
|
Histologic diagnosis*
|
|
|
Simple bone cyst |
1 (4.5) |
|
Aneurysmal bone cyst |
1 (4.5) |
|
Nonossifying fibroma |
3 (13.5) |
|
Enchondroma |
5 (22.5) |
|
Giant cell tumor |
2 (9.0) |
|
Lipoma |
1 (4.5) |
|
Fibrinoid necrosis |
2 (9.0) |
|
Fibrous dysplasia |
2 (9.0) |
|
Intraosseous lipoma |
3 (1.5) |
|
Follow-up period (mo) |
28.1 (24.1–38.4) |

The patients with lesions in the upper limbs were postoperatively immobilized in splints for 2 to 4 weeks. The patients with lesions in the lower limbs spent 4 to 6 weeks in non-weight-bearing splints. Full weight-bearing was allowed after 8 weeks.
To evaluate the resorption of β-TCP, a radiographic analysis was performed by the method of Hirata et al.
11) The length and width of the lesion filled with β-TCP were measured. Both clinical and radiological assessments were performed immediately after surgery and at the follow-up. The filling volume of β-TCP was measured using the following formula in radiographic analysis: (A × B
2) / 2, where B is the longest measured diameter, and A is the shorter one perpendicular to B. The resorption rate was calculated using the formula: (C − D) / C × 100, where C is the volume of β-TCP immediately after surgery and D is the volume at the time of final follow-up (
Fig. 1).
11) For statistical analysis of the data, we used Spearman's rank correlation coefficient. A
p-value less than 0.05 was considered to be statistically significant.
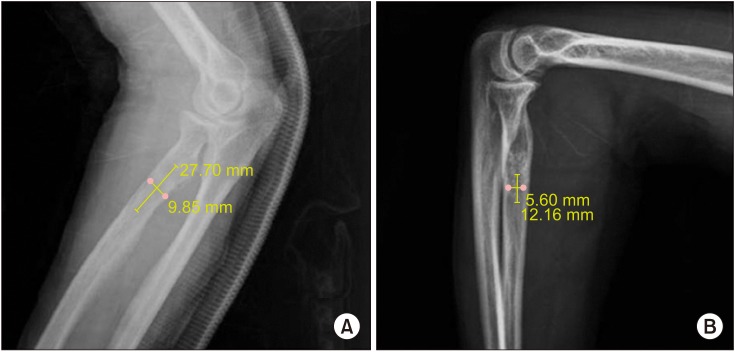 | Fig. 1Radiographs of a 57-year-old woman with intraosseous lipoma in the proximal radius. The filling volume of beta-tricalcium phosphate (β-TCP) was measured using the following formula: (A × B2) / 2, where A is the smaller diameter measured perpendicular to B, and B is the longest diameter. (A) Lateral view of the radius showing β-TCP injected immediately after surgery. The filling volume was 3.83 cm3. (B) Lateral view of the radius with complete resorption after 12 months. The filling volume was 0.43 cm3.
|
Go to :

RESULTS
Radiographically, the median filling volume of β-TCP immediately after operation was 23.8 cm
3 (range, 0.41 to 100.1 cm
3). When radiographically complete resorption was defined as greater than 50% resorption of the allograft material accompanied by bone remodeling until 12 months, there were 11 cases (55%) of complete resorption. (
Fig. 2). The median period for complete resorption of the material was 25.7 months (range, 25 to 26.3 months). The initial median filling volume after surgery was 9.7 cm
3 (range, 0.2 to 62.6 cm
3). Of the 16 patients older than 20 years, complete resorption was seen in eight (50%), whereas complete resorption was seen in three of the four patients younger than 20 years (75%). Positive correlation between the filling volume and time needed for complete resorption was not found (
p = 0.184) (
Fig. 3).
 | Fig. 2Radiographs of a 57-year-old woman with intraosseous lipoma in the proximal radius. Anteroposterior (A) and lateral (B) views of the radius showing beta-tricalcium phosphate (β-TCP) injected immediately after surgery. Anteroposterior (C) and lateral (D) views of the radius with complete resorption of β-TCP after 12 months. The resorption rate was 88.8%.
|
 | Fig. 3The graph shows the time taken for complete resorption according to filling volume. Correlation between the filling volume and time needed for complete resorption was not found (p = 0.184).
|
In nine cases (45%), radiographic signs of the grafted β-TCP remaining were noticed at the time of final follow-up, which was 24 months after surgery. However, the material in the bone marrow appeared to be incorporated and partially resorbed. Additionally, it was observed that the expanded cortex caused by tumor growth was restored to its original shape. Among these nine cases, the median filling volume was 54.0 cm3 (range, 0.4 to 100.1 cm3).
Go to :

DISCUSSION
Bone grafting is often required to manage defects generated from the surgical removal of tissues. Ideally, the substitute material should be biodegradable and fulfill certain requirements including biocompatibility, adequate initial strength and stiffness, retention of mechanical properties for sufficient time to assure biofunctionality, and nontoxicity of the degradation byproducts.
101213) β-TCP is one of the most commonly used calcium phosphate compounds as a synthetic bone-graft substitute.
910) This bioactive ceramic was deemed safe, nontoxic, safe from disease transmission, and free from immunogenic response. Most of all, it can be used without the risk of morbidity at the bone graft donor site. It is fully biocompatible and bioactive. Its synthetic origin ensures complete immune intolerance and absence of viral transmission.
7) β-TCP itself possesses an intrinsic osteoconductive property. Injection of β-TCP in combination with marrow-derived osteoprogenitor cells is reported to exhibit good osteogenic activity. Furthermore, handling and storage of β-TCP are relatively easy. Radiographically, the grafted β-TCP showed good incorporation into the host bone and induced new bone formation. In our study, there was no correlation between filling volume and time for complete resorption, which was contrary to the report of Nicholas and Lange
8) showing that healing was dependent upon defect size. This may be attributable to the small number of patients in our study. Galois et al.
7) mentioned that implant volume and patient's age were important factors affecting implant integration.
Research ensues on the use of β-TCP as a bone graft material in human subjects.
79) In our study, we achieved 55% of complete resorption in 12 months. This resorption rate is relatively superior to that in other studies.
11) We believe that purified β-TCP could be a suitable choice as a bone graft substitute especially after the removal of benign bone tumors.
Go to :

ACKNOWLEDGEMENTS
This study was supported by research fund from CGBio Inc. in 2015.
Go to :

Notes
Go to :

References
1. Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996; (329):300–309.

2. Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity: a statistical evaluation. Spine (Phila Pa 1976). 1995; 20(9):1055–1060. PMID:
7631235.
3. Dong J, Uemura T, Shirasaki Y, Tateishi T. Promotion of bone formation using highly pure porous beta-TCP combined with bone marrow-derived osteoprogenitor cells. Biomaterials. 2002; 23(23):4493–4502. PMID:
12322969.
4. Hollinger JO, Brekke J, Gruskin E, Lee D. Role of bone substitutes. Clin Orthop Relat Res. 1996; (324):55–65.

5. Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Rev Med Devices. 2006; 3(1):49–57. PMID:
16359252.

6. Bucholz RW. Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res. 2002; (395):44–52. PMID:
11937865.

7. Galois L, Mainard D, Delagoutte JP. Beta-tricalcium phosphate ceramic as a bone substitute in orthopaedic surgery. Int Orthop. 2002; 26(2):109–115. PMID:
12078872.
8. Nicholas RW, Lange TA. Granular tricalcium phosphate grafting of cavitary lesions in human bone. Clin Orthop Relat Res. 1994; (306):197–203.
9. Vaccaro AR. The role of the osteoconductive scaffold in synthetic bone graft. Orthopedics. 2002; 25(5 Suppl):s571–s578. PMID:
12038844.

10. Botez P, Sirbu P, Simion L, Munteanu F, Antoniac I. Application of a biphasic macroporous synthetic bone substitutes CERAFORM®: clinical and histological results. Eur J Orthop Surg Traumatol. 2009; 19(6):387–395.

11. Hirata M, Murata H, Takeshita H, Sakabe T, Tsuji Y, Kubo T. Use of purified beta-tricalcium phosphate for filling defects after curettage of benign bone tumours. Int Orthop. 2006; 30(6):510–513. PMID:
16736145.

12. Mano JF, Sousa RA, Boesel LF, Neves NM, Reis RL. Bioinert, biodegradable and injectable polymeric matrix composites for hard tissue replacement: state of the art and recent developments. Compos Sci Technol. 2004; 64(6):789–817.

13. Naito K, Obayashi O, Mogami A, Itoi A, Kaneko K. Fracture of the calcium phosphate bone cement which used to enchondroma of the hand: a case report. Eur J Orthop Surg Traumatol. 2008; 18(5):405–408.

Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download