Abstract
Background
Spinal diseases are self-limited or non-progressive in many cases. Epidural steroid injection (ESI) is a common nonsurgical treatment option for spinal pain. Despite concerns about complications of repeated steroid injection, few studies reported on the adrenal function of spine disease patients undergoing surgery after ESI. We investigated the influence of preoperative multiple ESIs on adrenal function in spine surgery patients.
Methods
This was a retrospective study with prospective data collection. Those who underwent elective spinal operations and had a history of multiple ESIs from January to June 2017 were selected as a study group. Those who underwent knee arthroplasty and did not have a history of ESI and any kind of steroid injection in other areas during 6 months before surgery were selected as a control group. Demographic data were compared to assess homogeneity between groups. We assessed the preoperative serum cortisol level (SCL) to compare the basal adrenal function between groups. Also, we assessed the elevation of SCL postoperatively to evaluate the adrenal response to the surgical stress in each group. For subgroup analysis, we divided all patients into normal (7–28 µg/dL) and subnormal groups according to SCL and analyzed risk factors of adrenal suppression with multivariate logistic regression test.
Results
There were 53 patients in the study group and 130 in the control group. Age and sex were homogeneous between groups. There was significant intergroup difference in preoperative SCL (10.4 ± 4.8 µg/dL in the study group vs. 12.0 ± 4.2 µg/dL in the control group; p = 0.026).The postoperative day one SCL was 11.6 ± 5.0 µg/dL in the study group without significant increase from the preoperative level (p = 0.117), whereas the increase was significant in the control group with a postoperative level of 14.4 ± 4.4 µg/dL (p < 0.001). Among all patients, the SCL was subnormal in 18 patients and within the normal range in 165. Spine surgery was the independent risk factor irrespective of age and sex (odds ratio, 3.472; p = 0.015).
Epidural steroid injection (ESI) has been widely used in various spinal diseases and its efficacy is generally accepted for management of acute and chronic radicular pain.123) Although its long-term efficacy has not yet been established, itis well recognized as a cost-effective procedure.4) However, concerns about the complications related to the procedure have also been raised,567) although systemic adverse effects of steroid injection were reported in only a few cases.891011) In Korea, surgical treatment of degenerative spinal diseases is strongly regulated by the state-run medical insurance company. Therefore, more spinal disease patients are receiving ESI recently.12) Although overt Cushing's syndrome caused by ESI is rare, hypothalamus-pituitary-adrenal (HPA) axis suppression after ESI is suspected to be common. We also encountered patients who were suffering more from symptoms suggestive of adrenal suppression after spinal surgery than patients who underwent other major orthopedic operations of similar magnitude did. Therefore, we hypothesized that patients who receive spinal surgery due to degenerative disease have adrenal insufficiency and their adrenal response to surgical stress is suppressed by multiple ESIs.
Patients with degenerative disease who underwent spinal surgery at Seoul Sacred Heart General Hospital from January 2017 to June 2017 were enrolled as a study group (n = 53). Patients who underwent joint arthroplasty at the same period and had no history of steroid injection based on patient's self-assessment form were enrolled as a control group (n = 130). Informed consent was obtained from all patients. We examined the preoperative serum cortisol level (SCL) at 6:00 am on the operation day and the postoperative SCL at 6:00 am on postoperative day 1. The data were reviewed retrospectively. Patients who had infections or trauma or took steroid for various reasons were excluded from the study. We compared the preoperative SCL between the two groups. We also compared the postoperative change in SCL from the preoperative level in each group to assess the adrenal response to the surgical stress. To investigate the independent risk factors for adrenal suppression, we divided all patients according to the SCL in to a depression group (< 7 µg/dL) and a normal group (7–28 µg/dL) and analyzed all parameters.
For statistical analysis, demographic homogeneity between the two groups were analyzed by Student t-test for a parametric variable and Fisher exact test for a nonparametric variable. Preoperative SCL was compared using a Student t-test and postoperative change was compared using a paired t-test. Risk factor analysis was done by multivariate logistic regression analysis. We used SPSS ver. 14.0 (SPSS Inc., Chicago, IL, USA) for all statistical analysis.
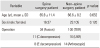
There were 53 patients in the study group and 130 patients in the control group who met the inclusion criteria. There was no significant demographic difference (with regard to age and sex) between the two groups (Table 1).
The preoperative SCL was 10.4 ± 4.8 µg/dL in the study group and 12.0 ± 4.2 µg/dL in the control group; the difference was significant (p = 0.026). In the study group, the postoperative day 1 SCL was 11.6 ± 5.0 µg/dL, and postoperatively there was no significant increase form the preoperative level (p = 0.117). By contrast, the postoperative level was 14.4 ± 4.4 µg/dL in the control group, indicating a significant rise in the SCL level (p < 0.001) (Table 2).
The SCL was not higher than the upper normal limit in any of the patients. Among all patients, the SCL was subnormal in 18 patients and within the normal range in 165 patients. In the multivariate logistic regression model, spine surgery was the only risk factor for subnormal SCL (odds ratio [OR], 3.472; p = 0.015).
Even after the surgical stress, the SCL was not higher than the upper normal limit in all patients. Among all patients, the SCL was subnormal in nine patients and within the normal range in 174 patients. In the multivariate logistic regression model, spine surgery was the only risk factor for subnormal SCL (OR, 9.421; p = 0.007).
ESI is widely accepted as an effective method for management of radiculopathy and is one of the most commonly performed procedures in pain clinics.12) Although most physicians assume that it is a safe procedure with only a few negligible complications, concerns about systemic adverse effects of ESI, such as metabolic,13) vascular,14) cardiopulmonary,15) lipomatosis,16) visual,9) dysphonia,17) and endocrine effects, have been consistently raised. In Korea, nowadays, all patients with spine diseases that may require surgery should receive intensive conservative treatments for a while regardless of their disease entities to receive reimbursement from the state-run insurance company. Therefore, the frequency of ESI before a surgical treatment has been increasing rapidly. We have encountered a growing number of patients who suffer from loss of appetite after spine surgery in recent days. Cushing's syndrome secondary to ESI is very rare;1819) however, HPA axis suppression after ESI is much more common and affects for a long period. Even after a single ESI containing 40 mg/mL or 80 mg/mL methylprednisolone, HPA axis was suppressed for more than 3 weeks.20) In the case report of Tuel et al.,19) normalization of HPA axis function was obtained by 6 months after a single ESI of 60 mg/mL methylprednisolone. The duration of suppression is supposed to increase as the frequency of ESI increases.11) However, there is no consensus on the safe frequency of ESI; no study has specifically explored this subject. Some methodologically limited studies suggested that repeat injections may improve outcomes, but the evidence is insufficient to make any definite conclusions.21)
At first we compared the early morning (6:00 am) SCLs between the two groups. Then, postoperative day 1 early morning SCLs were measured and compared with preoperative values in each group. We intended to evaluate the adrenal function of spine surgery patients who underwent ESIs. As we expected, the preoperative SCL was lower, and adrenal response to surgical stress was also more suppressed in the spine surgery patients than in the control group. To verify the independency of spine surgery as a risk factor for adrenal suppression and poor reaction to surgical stress, we divided all patients into normal and subnormal SCL groups and applied the multivariate logistic regression analysis. The spine surgery was the only risk factor for subnormal SCL preoperatively and postoperatively. This can be interpreted that treatment for spine surgery does not only include the operation itself but also the preoperative nonsurgical procedures. Many spine surgery patients seem to suffer more than patients with other operations do from general weakness, pain, and loss of appetite, which can be attributed to HPA axis suppression. Most degenerative spinal diseases are known to be well improved by ESI. Although there are some refractory cases, considering the overt adverse effects on the HPA axis, we should take care to avoid repeated ESIs.
Most previous data and research are based on particulate steroids like methylprednisolone and triamcinolone that have larger diameters than those of red blood cells.2223) However, particulate steroids are no longer used for ESI because of the potential risk of arterial embolism. Only nonparticulate steroid, dexamethasone, is available nowadays. Its half-time is much longer than that of particulate steroids,23) so we can presume that HPA axis suppression would be extended with use of dexamethasone. To the best of our knowledge, there is no research that compares the duration of efficacy of dexamethasone and other particulate steroids. According to the study of Friedly et al.,24) all kinds of steroids available (methylprednisolone, betamethasone, triamcinolone, and dexamethasone) lower the SCL for at least more than 3 weeks. Roberts et al.25) reviewed transforaminal epidural injections and concluded that transforaminal injection with bupivacaine or saline only was as good as transforaminal steroid injection. It suggests that steroid can be excluded in epidural injection therapy.
There are some limitations to this study. In the study group, the number of ESIs and specific dosage and regimen were not considered. In the control group, their previous history of steroid use was entirely dependent on the patients' memory. In addition, the magnitude of surgical stress according to the kind of operations was not taken into account. For the exact evaluation of secondary adrenal insufficiency, adrenocorticotropic hormone (ACTH) stimulation test and serum ACTH level assessment are necessary, which were not examined in this study. On the analysis of risk factors for adrenal suppression, the number of patients was not sufficient and entered variables were too simple to consider the comprehensive risk in the complicated senior patients. Nevertheless, to the best of our knowledge, this is the first pilot study to raise awareness about the potential endocrinological adverse effects of ESI in the real clinical setting of spine surgery patients. We hope our study will spur more research into the influence of ESI on adrenal function with well-designed and prospective studies.
Figures and Tables
References
1. Benyamin RM, Manchikanti L, Parr AT, et al. The effectiveness of lumbar interlaminar epidural injections in managing chronic low back and lower extremity pain. Pain Physician. 2012; 15(4):E363–E404.
3. Manchikanti L, Buenaventura RM, Manchikanti KN, et al. Effectiveness of therapeutic lumbar transforaminal epidural steroid injections in managing lumbar spinal pain. Pain Physician. 2012; 15(3):E199–E245.
4. Whynes DK, McCahon RA, Ravenscroft A, Hardman J. Cost effectiveness of epidural steroid injections to manage chronic lower back pain. BMC Anesthesiol. 2012; 12:26.

5. Lee JW, Lee E, Lee GY, Kang Y, Ahn JM, Kang HS. Epidural steroid injection-related events requiring hospitalisation or emergency room visits among 52,935 procedures performed at a single centre. Eur Radiol. 2018; 28(1):418–427.

6. McGrath JM, Schaefer MP, Malkamaki DM. Incidence and characteristics of complications from epidural steroid injections. Pain Med. 2011; 12(5):726–731.

7. Malhotra G, Abbasi A, Rhee M. Complications of transforaminal cervical epidural steroid injections. Spine (Phila Pa 1976). 2009; 34(7):731–739.

8. Abdul AJ, Ghai B, Bansal D, Sachdeva N, Bhansali A, Dhatt SS. Hypothalamic pituitary adrenocortical axis suppression following a single epidural injection of methylprednisolone acetate. Pain Physician. 2017; 20(7):E991–E1001.
9. Browning DJ. Acute retinal necrosis following epidural steroid injections. Am J Ophthalmol. 2003; 136(1):192–194.

10. Horani MH, Silverberg AB. Secondary Cushing's syndrome after a single epidural injection of a corticosteroid. Endocr Pract. 2005; 11(6):408–410.

11. Jacobs S, Pullan PT, Potter JM, Shenfield GM. Adrenal suppression following extradural steroids. Anaesthesia. 1983; 38(10):953–956.

12. Manchikanti L, Pampati V, Falco FJ, Hirsch JA. Growth of spinal interventional pain management techniques: analysis of utilization trends and Medicare expenditures 2000 to 2008. Spine (Phila Pa 1976). 2013; 38(2):157–168.
13. Even JL, Crosby CG, Song Y, McGirt MJ, Devin CJ. Effects of epidural steroid injections on blood glucose levels in patients with diabetes mellitus. Spine (Phila Pa 1976). 2012; 37(1):E46–E50.

14. DeSio JM, Kahn CH, Warfield CA. Facial flushing and/or generalized erythema after epidural steroid injection. Anesth Analg. 1995; 80(3):617–619.

15. Stauber B, Ma L, Nazari R. Cardiopulmonary arrest following cervical epidural injection. Pain Physician. 2012; 15(2):147–152.
16. Gupta R, Shah M, Reese CM. Steroid induced spinal epidural lipomatosis: case report and review of the literature. W V Med J. 2011; 107(4):20–22.
17. Slipman CW, Chow DW, Lenrow DA, Blaugrund JE, Chou LH. Dysphonia associated with epidural steroid injection: a case report. Arch Phys Med Rehabil. 2002; 83(9):1309–1310.

18. Stambough JL, Booth RE Jr, Rothman RH. Transient hypercorticism after epidural steroid injection: a case report. J Bone Joint Surg Am. 1984; 66(7):1115–1116.

19. Tuel SM, Meythaler JM, Cross LL. Cushing's syndrome from epidural methylprednisolone. Pain. 1990; 40(1):81–84.

20. Habib G, Jabbour A, Salman J, Hakim G, Haddad H. The effect of epidural methylprednisolone acetate injection on the hypothalamic-pituitary-adrenal axis. J Clin Anesth. 2013; 25(8):629–633.

21. Novak S, Nemeth WC. The basis for recommending repeating epidural steroid injections for radicular low back pain: a literature review. Arch Phys Med Rehabil. 2008; 89(3):543–552.

22. Derby R, Lee SH, Date ES, Lee JH, Lee CH. Size and aggregation of corticosteroids used for epidural injections. Pain Med. 2008; 9(2):227–234.

23. Benzon HT, Chew TL, McCarthy RJ, Benzon HA, Walega DR. Comparison of the particle sizes of different steroids and the effect of dilution: a review of the relative neurotoxicities of the steroids. Anesthesiology. 2007; 106(2):331–338.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download