This article has been
cited by other articles in ScienceCentral.
Abstract
Background
The incidence of facet tropism (FT) and its correlation with low back pain (LBP) have, to our knowledge, not yet been investigated among selected community-based populations who visited departments unrelated to LBP with their chief complaints unrelated to LBP. In this study, we aimed to evaluate the prevalence of FT among selected patients in whom LBP was not the chief complaint and the correlation between FT and LBP among these patients.
Methods
Among patients who underwent computed tomography during 2014 for reasons unrelated to LBP, we enrolled 462 patients who satisfied the inclusion and exclusion criteria. The degree of tropism was defined as grade 0, 1, and 2 for FT, FT+, and FT++, respectively. LBP was evaluated using a modified version of the Nordic low back pain questionnaire. For additional evaluation of dynamic LBP, the question, “Did your pain go away when lying down still or standing up straight, and did it also intensify when you bend or stretch your back?,” was included in the questionnaire.
Results
The L4–5 intervertebral area was most frequently and severely affected by FT with an incidence rate of 46.3%, and severe FT was observed in 24.7% of the patients. FT increased with age at L2–3 and L5–S1 levels. FT at L2–3 level was correlated with LBP (p = 0.035) and dynamic LBP (p = 0.033). The FT grade at L2–3 level was correlated with dynamic LBP (p = 0.022) but not with LBP (p = 0.077). The relative risk of FT at L2–3 level was 1.614 for LBP and 1.724 for dynamic LBP.
Conclusions
The prevalence of FT among community-based populations was 46.3% and its severe form was more frequently observed at L4–5 level (24.7%). LBP was correlated with FT at L2–3 level. The relative FT-associated risk of LBP was 1.6 at L2–3 level, and the relative L2–3 FT-associated risk of dynamic LBP was 1.724.
Go to :

Keywords: Low back pain, Lumbar vertebrae, Zygapophyseal joint, Tropism
The facet joint is the only synovial joint comprising the articular capsule, synovial membrane, and the hyaline cartilage that covers the subchondral bone.
12) Facet tropism (FT) is defined as the asymmetry between the sagittal angle of the left and right facet joints.
34) FT causes torsion during lumbar flexion and extension and accordingly triggers spinal instability and pathologic changes in the vertebral disc.
3) Van Schaik et al.
5) have established the relationship between low back pain (LBP) and intervertebral disc herniation at L4–5 level in 100 patients with LBP 30 years ago. Other authors have insisted that the broad movement of the facet joint or the high load to the facet joint lead to degenerative changes, which is the actual cause of LBP.
6) Degenerative changes of the facet joint are known to be associated with age, facet orientation, and degenerative changes of the intervertebral disc.
178) In addition, recent studies have focused on the relationship between FT and degenerative changes of the intervertebral disc, herniated disc, or discogenic pain.
9) Some authors insist that torsion caused by FT triggers LBP during rotation toward the most oblique facet joint.
3)
However, most studies have been conducted by departments associated with LBP on patients with symptomatic LBP. This can result in selection bias and overestimation of the prevalence of FT. Although some authors have set the control group as normal individuals, the patient group consisted of patients with LBP. Studies on patients who underwent abdominal computed tomography (CT) for complaints other than LBP are limited. To the best of our knowledge, our study is the largest and first to estimate FT incidence and evaluate its correlation with LBP among patients who visited departments unrelated to LBP (not the departments of orthopedic surgery, neurosurgery, rehabilitation medicine, pain medicine, and rheumatology).
The purposes of the present study were to evaluate the prevalence of FT by vertebral levels and to identify the relationship between FT and LBP in selected community-based populations with chief complaints other than LBP.
METHODS
Patients
This is a prospective cross-sectional study with level 3 evidence. During 2014, among all patients who underwent abdominal CT, not CT of the lumbar spine, we enrolled 462 patients who satisfied the following inclusion criteria: patients aged 40–80 years, visited departments unrelated to LBP (such as the department of general surgery or urology, but not the departments of orthopedic surgery, neurosurgery, rehabilitation medicine, and rheumatology), and underwent CT due to complaints unrelated to LBP. Patients were excluded if they had already been diagnosed with spinal disorders such as scoliosis, spondylolisthesis, spinal tumor, spinal tuberculosis, infectious spondylitis, and congenital anomalies; had received treatment for spinal disorders; or had undergone spinal operations. We conducted this study in compliance with the principles of the Declaration of Helsinki. The protocol of this study was reviewed and approved by the Institutional Review Board of Daegu Catholic University Medical Center (IRB No. CR-16-167). Written informed consents were obtained.
Evaluation of LBP
Before CT scanning, the degree of LBP was evaluated in all patients by impartial skilled nurses. According to a modified version of Nordic low back pain questionnaire,
10) patients were asked to respond with “yes” or “no” to the question, “Have you at any time during the last 12 months received medical treatment every day, for at least a month, for low back pain?” Additionally, for the evaluation of dynamic LBP, patients who responded with “yes” to the above question were asked to respond with “yes” or “no” to the question, “Did your pain go away when lying down still or standing up straight, and did it also intensify when you bend or stretch your back?”
Facet Joint Tropism Evaluation
The evaluation of FT was independently and blindly performed by two orthopedic surgeons (SK and SC). Using the secure-access picture-archiving communication system (PACS; Philips Sectra, Linköping, Sweden), the axial view of each intervertebral space was obtained at the midpoint of the intervertebral discs. In total, 3,696 facet joints obtained from 462 patients underwent evaluation. Facet orientation was measured at the midpoint of each vertebral level between L2 and S1. Asymmetry was evaluated by measuring the difference between the orientations of bilateral facet joints. The orientation of the facet joints was measured according to the method described by Noren et al.
11) using the axial view from CT scans at the L2–3, L3–4, L4–5, and L5–S1 levels. After setting the rear portion of the vertebral body as a datum point, we drew a line vertically through the middle of the spinous process. The angle between this “facet line” and the coronal plane of each facet joint was defined as the facet angle (
Fig. 1). Asymmetry was defined as the absolute value of difference between the left and right facet angles. The description of FT differs among authors. For example, Vanharanta et al.
9) defined FT as a difference of > 7° between the two facet angles. Prabhoo et al.
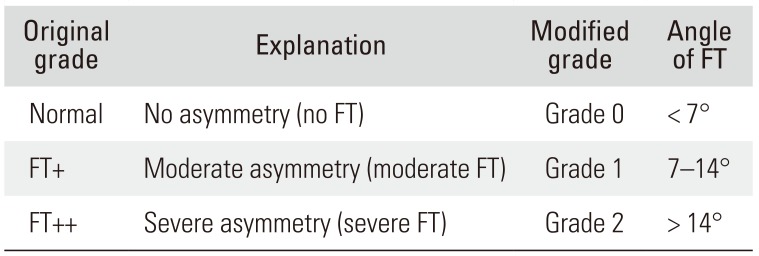
12) calculated the mean and standard deviation using the asymmetric distribution of the L4–5 facet joint. We have modified the classification method by Vanharanta et al.
9) into grades 0, 1, and 2 for FT, FT+, and FT++ (
Table 1). Patients with grades 1 or 2 were considered to have FT.
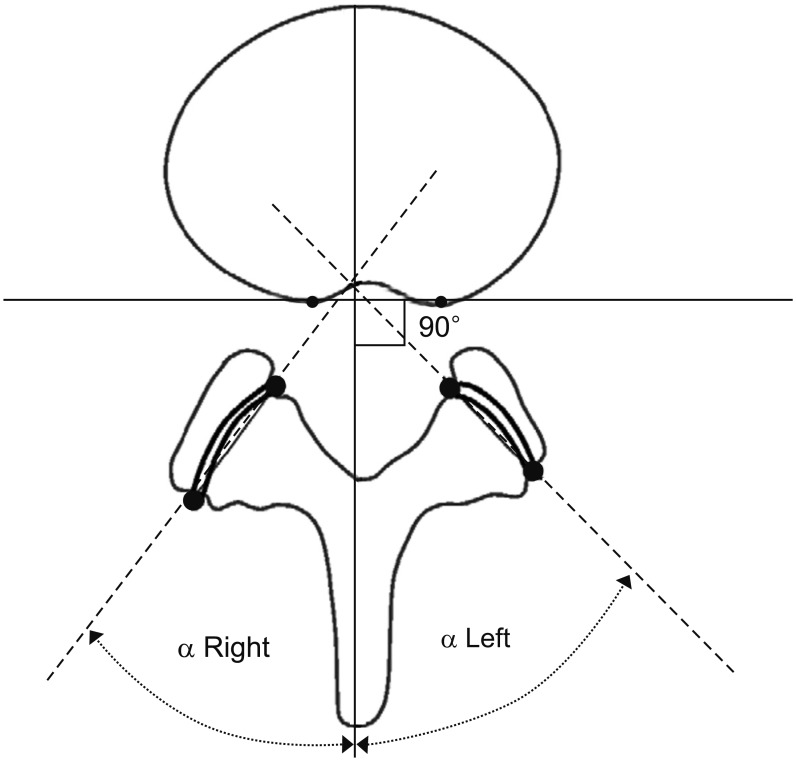 | Fig. 1After setting the rear portion of the vertebral body as a datum point, we drew a vertical line through the middle of the spinous process. The angle between this “facet line” and the coronal plane of each facet joint was defined as the facet angle.
|
Table 1
Modification of FT
|
Original grade |
Explanation |
Modified |
Angle of FT |
|
Normal |
No asymmetry (no FT) |
Grade 0 |
< 7° |
|
FT+ |
Moderate asymmetry (moderate FT) |
Grade 1 |
7–14° |
|
FT++ |
Severe asymmetry (severe FT) |
Grade 2 |
> 14° |

Scanning Parameters
CT was performed using the 16-multidetector CT (MDCT) or the dual source 64-channel MDCT (Light-speed Ultra; GE Healthcare, Milwaukee, WI, USA). Axial films were cut into 2-mm-thick sections, to fit each department's protocol.
Statistical Analysis
All statistical analyses were performed using IBM SPSS ver. 19.0 (IBM Corp., Armonk, NY, USA). The relationship between LBP and FT was evaluated using Pearson chi-square test. The relationship between age and FT was evaluated using analysis of variance. The relative risk conferred by FT on LBP was evaluated using a multiple logistic regression test. A p-value < 0.05 was considered statistically significant.
Go to :

RESULTS
Epidemiological Results
Among the 462 participants, 258 were men and 204 were women. The average age among participants was 59.02 ± 15.24 years (range, 40 to 80 years). The average age of male participants was 59.38 ± 15.03 years, and that of female participants was 58.57 ± 15.31 years. The two groups showed no significant statistical differences (p = 0.568). Out of 462 patients, 207 were in general surgery, 127 in urology, 86 in digestive medicine, and 42 in obstetrics and gynecology.
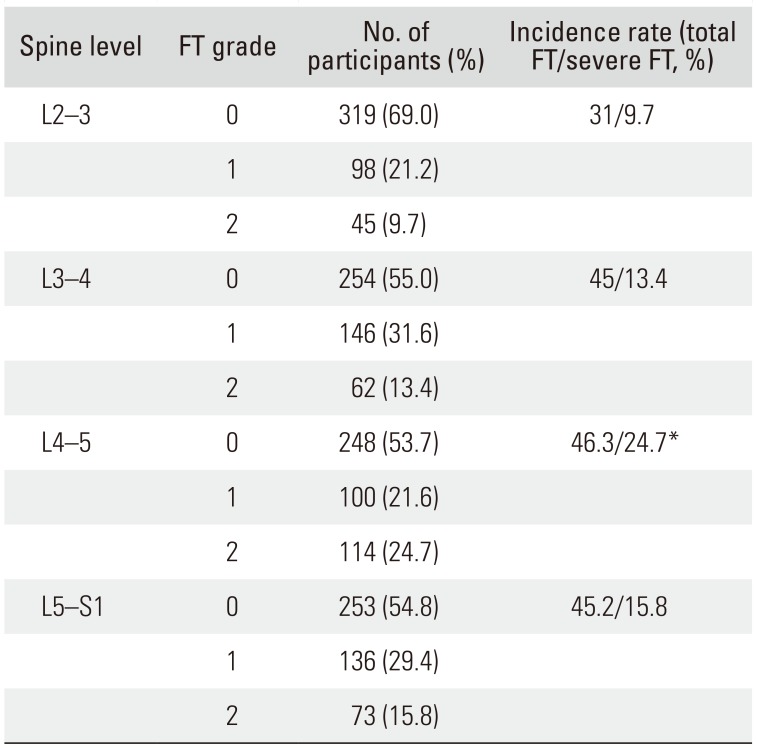
Prevalence of FT
At the L2–3 level, there were 319 patients with FT grade 0, 98 patients with FT grade 1, and 45 patients with FT grade 2. The incidence rates of FT and severe FT were 31% and 9.7%, respectively. At the L3–4 level, there were 254 patients with FT grade 0, 146 patients with FT grade 1, and 62 patients with FT grade 2. The incidence rates of FT and severe FT among these patients were 45% and 13.4%, respectively. At the L4–5 level, there were 248 patients with FT grade 0, 100 patients with FT grade 1, and 114 patients with FT grade 2. The incidence rates of FT and severe FT were 46.3% and 24.7%, respectively, showing the highest incidence rate of severe FT among all vertebral levels (
p < 0.05). At the L5–S1 level, there were 253 patients with FT grade 0, 136 patients with FT grade 1, and 73 patients with FT grade 2. The incidence rates of FT and severe FT were 45.2% and 15.8%, respectively. While the prevalence of FT did not differ significantly between vertebral levels, the incidence of severe FT was significantly higher at the L4–5 level (
p < 0.05) (
Table 2).
Table 2
Prevalence of FT
|
Spine level |
FT grade |
No. of participants (%) |
Incidence rate (total FT/severe FT, %) |
|
L2–3 |
0 |
319 (69.0) |
31/9.7 |
|
1 |
98 (21.2) |
|
|
2 |
45 (9.7) |
|
|
L3–4 |
0 |
254 (55.0) |
45/13.4 |
|
1 |
146 (31.6) |
|
|
2 |
62 (13.4) |
|
|
L4–5 |
0 |
248 (53.7) |
46.3/24.7*
|
|
1 |
100 (21.6) |
|
|
2 |
114 (24.7) |
|
|
L5–S1 |
0 |
253 (54.8) |
45.2/15.8 |
|
1 |
136 (29.4) |
|
|
2 |
73 (15.8) |
|

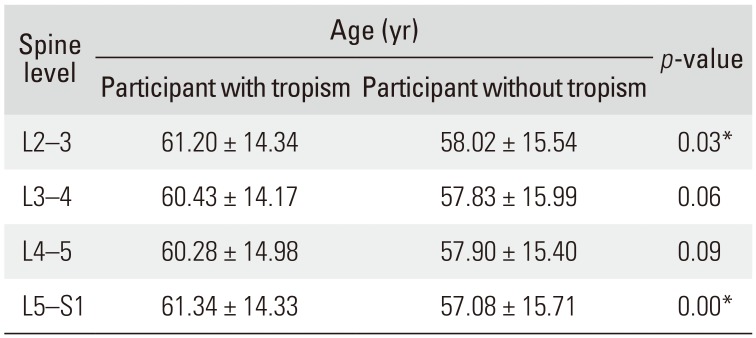
Correlation between FT and Age
A significant correlation between age and FT was observed at the L2–3 and L5–S1 levels (both
p < 0.05). However, at L3–4 and L4–5 levels, a statistically significant correlation between age and FT was not observed (
p = 0.06 and
p = 0.09, respectively) (
Table 3).
Table 3
Correlation between Facet Tropism and Age
|
Spine level |
Age (yr) |
p-value |
|
Participant with tropism Participant without tropism |
|
L2–3 |
61.20 ± 14.34 |
58.02 ± 15.54 |
0.03*
|
|
L3–4 |
60.43 ± 14.17 |
57.83 ± 15.99 |
0.06 |
|
L4–5 |
60.28 ± 14.98 |
57.90 ± 15.40 |
0.09 |
|
L5–S1 |
61.34 ± 14.33 |
57.08 ± 15.71 |
0.00*
|

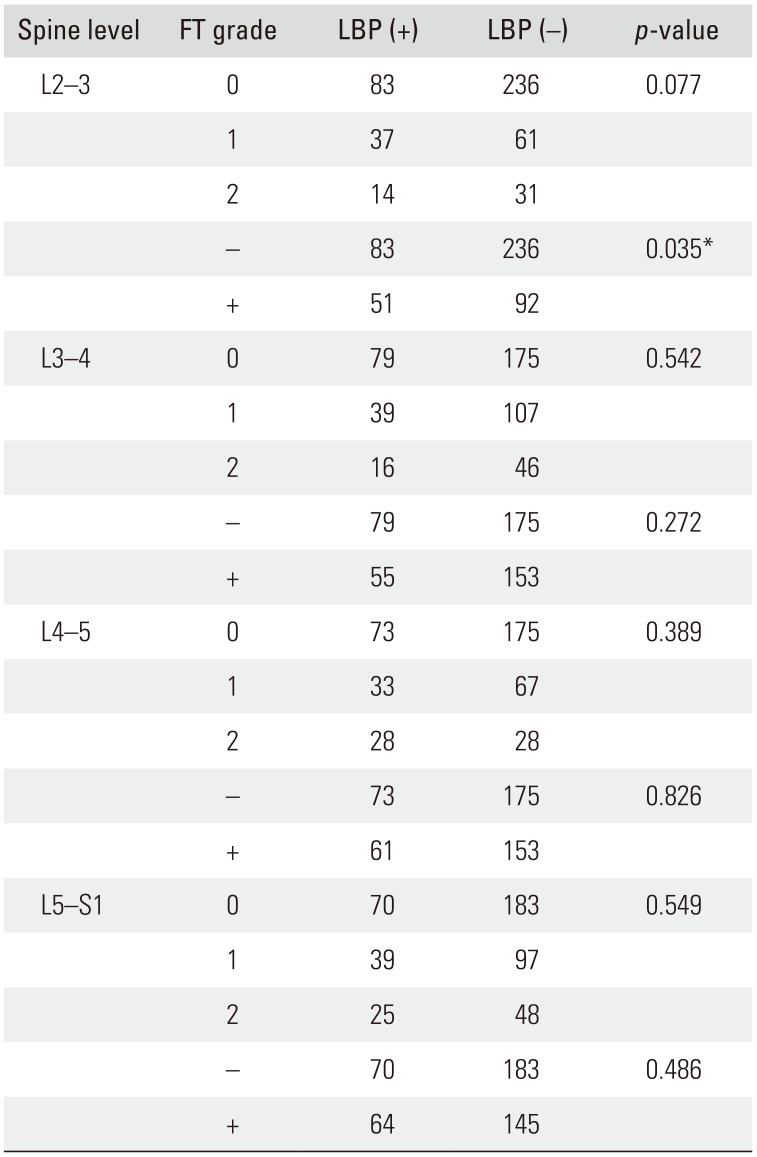
Correlation between LBP and FT
Among the 462 patients, 134 patients had LBP and 328 did not. LBP and FT grade were not correlated at L2–3 level (
p = 0.077). However, LBP and the presence of FT was correlated at the L2–3 level (
p = 0.035). At the L3–4, L4–5, and L5–S1 levels, LBP and FT grade were not correlated (
p = 0.542,
p = 0.389, and
p = 0.549, respectively). Additionally, no correlation was noted between LBP and FT at other vertebral levels (L3–4,
p = 0.272; L4–5,
p = 0.826; and L5–S1,
p = 0.486, respectively) (
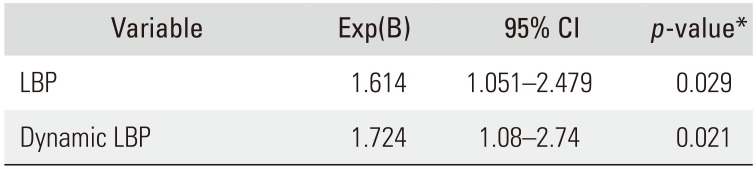
Table 4). The relative risk of FT-associated LBP at L2–3 was 1.614 according to multiple logistic regression analysis (
p = 0.029) (
Table 5).
Table 4
Correlation between LBP and FT
|
Spine level |
FT grade |
LBP (+) |
LBP (−) |
p-value |
|
L2–3 |
0 |
83 |
236 |
0.077 |
|
1 |
37 |
61 |
|
|
2 |
14 |
31 |
|
|
− |
83 |
236 |
0.035*
|
|
+ |
51 |
92 |
|
|
L3–4 |
0 |
79 |
175 |
0.542 |
|
1 |
39 |
107 |
|
|
2 |
16 |
46 |
|
|
− |
79 |
175 |
0.272 |
|
+ |
55 |
153 |
|
|
L4–5 |
0 |
73 |
175 |
0.389 |
|
1 |
33 |
67 |
|
|
2 |
28 |
28 |
|
|
− |
73 |
175 |
0.826 |
|
+ |
61 |
153 |
|
|
L5–S1 |
0 |
70 |
183 |
0.549 |
|
1 |
39 |
97 |
|
|
2 |
25 |
48 |
|
|
− |
70 |
183 |
0.486 |
|
+ |
64 |
145 |
|

Table 5
The Relative Risk of L2–3 Tropism by Multiple Logistic Regression Analysis
|
Variable |
Exp(B) |
95% CI |
p-value*
|
|
LBP |
1.614 |
1.051–2.479 |
0.029 |
|
Dynamic LBP |
1.724 |
1.08–2.74 |
0.021 |

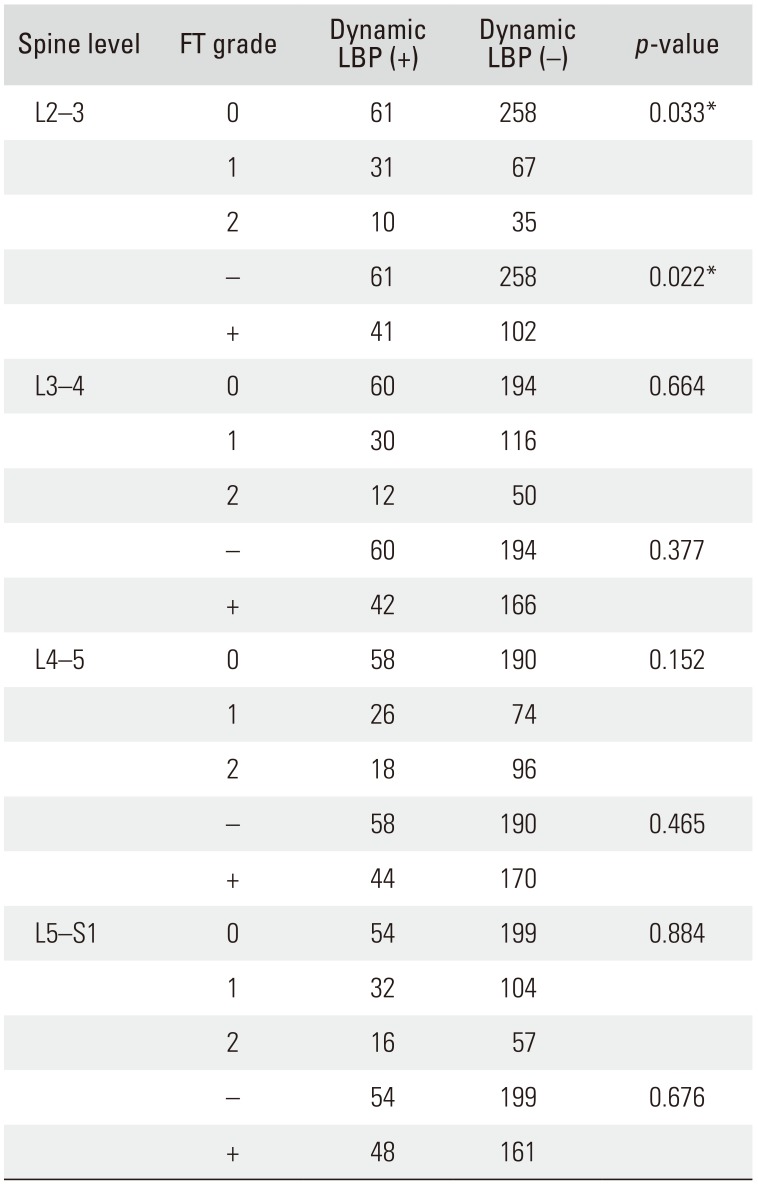
Correlation between FT and Dynamic LBP among LBP Patient Groups
Among the 462 patients, 102 patients had dynamic LBP and 360 patients did not. FT grade and dynamic LBP were significantly correlated at the L2–3 level (
p = 0.033); however, no significant correlation was observed at the L3–4, L4–5, and L5–S1 levels (
p = 0.664,
p = 0.152, and
p = 0.884, respectively). Although FT was significantly correlated with LBP at the L2–3 level (
p = 0.022), there was no correlation between the two at L3–4, L4–5, and L5–S1 levels (
p = 0.377,
p = 0.465, and
p = 0.676, respectively) (
Table 6). The relative risk of FT-associated dynamic LBP at L2–3 was 1.724 according to multiple logistic regression analysis (
p = 0.021) (
Table 5).
Table 6
Correlation between Dynamic LBP and FT
|
Spine level |
FT grade |
Dynamic LBP (+) |
Dynamic LBP (−) |
p-value |
|
L2–3 |
0 |
61 |
258 |
0.033*
|
|
1 |
31 |
67 |
|
|
2 |
10 |
35 |
|
|
− |
61 |
258 |
0.022*
|
|
+ |
41 |
102 |
|
|
L3–4 |
0 |
60 |
194 |
0.664 |
|
1 |
30 |
116 |
|
|
2 |
12 |
50 |
|
|
− |
60 |
194 |
0.377 |
|
+ |
42 |
166 |
|
|
L4–5 |
0 |
58 |
190 |
0.152 |
|
1 |
26 |
74 |
|
|
2 |
18 |
96 |
|
|
− |
58 |
190 |
0.465 |
|
+ |
44 |
170 |
|
|
L5–S1 |
0 |
54 |
199 |
0.884 |
|
1 |
32 |
104 |
|
|
2 |
16 |
57 |
|
|
− |
54 |
199 |
0.676 |
|
+ |
48 |
161 |
|

Go to :

DISCUSSION
Do et al.
13) reported a mild FT prevalence of 28% at L3–4 level, 28% at L4–5, and 24% at L5–S1, and a severe FT prevalence of 14% at L3–4 level, 15% at L4–5, and 17% at L5–S1, showing no significant difference by the severity of FT. Kalichman and Hunter
1) reported an FT (≥ 7°) prevalence of 76.7% in men and 66.3% in women and there was no statistically significant difference at any vertebral level. In the present study, we reported an overall FT prevalence of 45% and a severe FT prevalence of 24.7%, mostly affecting vertebral L4–5 level. The difference in FT prevalence reported by Do et al.
13) and Kalichman et al.
14) may be attributed to demographic differences; both studies enrolled patients with spinal disorders.
Kalichman et al.
14) reported that FT was not associated with age, while Do et al.
13) reported that FT showed some association with age at the L4–5 level but not at the L3–4 and L5–S1 levels. In this study, we found some correlation between FT and age at L2–3 and L5–1 levels but no correlation at L3–4 and L4–5 levels. This difference may be attributed to differing patient characteristics, such as the vertical orientation of the facet joint or racial differences. Further studies are required to understand these differences. LBP was evaluated by Holmstrom and Moritz's questionnaire.
15) Before CT scanning, functional assessment was performed using the modified Nordic low back pain questionnaire,
10) which is known for its high clinical correlation.
Variation in the facet angle measured using CT has been reported.
16) Facet joint orientation in the horizontal plane reportedly has 10°–15° of standard deviation in Caucasian and African American bone models
17) and in patients with spondylolisthesis in the Asia-Pacific region,
18) which suggests the presence of a wide variation between individuals. Furthermore, facet joint orientation in the horizontal plane changes toward the sagittal direction with aging.
16) Although the difference in both facet angles is < 7° at the lumbar spine, the difference may vary up to 70°. Grogan et al.
19) and Vanharanta et al.
9) suggested classification of facet joint angulation.
Liu et al.
2) and Fujiwara et al.
7) have insisted that FT is associated with degenerative osteoarthritis of the facet joint and may play a role in LBP: degenerative changes of the facet joint may worsen LBP during the flexion and extension of the back, which involves movement of the facet joint. Consequently, severe FT at the L4–5 level where movement is most intense may play a crucial role in dynamic LBP.
8) However, studies have also reported no correlation between FT and degenerative changes of the facet joint.
714) Another hypothetical mechanism proposed by Liu et al.
2) is that degenerative changes cause abnormal force to the facet joint, and thus morphological changes may occur in the facet joint as a result of bone remodeling in degenerative osteoarthritis. However, this mechanism is just hypothetical and has yet to be verified.
Although FT is considered a major cause of LBP, the exact pathophysiology remains unclear.
20) Several authors have reported that radiological asymmetry in the facet joints is associated with LBP and LBP-causing diseases. Cyron and Hutton
21) reported in a biomechanical study that FT increases the shear force on the spine and eventually poses as a risk factor for LBP. While Berlemann et al.
22) and Dai
23) reported a correlation between FT and spondylolisthesis and Karacan et al.
4) reported a correlation between FT and herniated nucleus pulposus, studies on causality show conflicting results.
1237141924)
Farfan and Sullivan
25) reported that tear patterns of the posterior annulus appeared to be related to facet joint asymmetry and that there was a high association between the side of disc herniation and the more coronally facing facet joint. Van Schaik et al.
5) showed correlations between LBP at the L4–5 level and disc herniation in a study on 100 patients with LBP. Recently, Do et al.
13) reported a correlation between severe FT at L4–5 level and disc bulging in old age; however, the relationship between LBP and disc bulging remains controversial.
In the present study, the relative risk of FT-associated LBP at L2–3 was 1.614, and the relative FT-associated risk of dynamic LBP at L2–3 was 1.724. FT in other spine levels showed no association with LBP and dynamic LBP. This may be due to selection bias since other studies have not taken FT at L2–3 into account.
Only a limited number of studies have focused on the association between FT at L2–3 and LBP. On the basis of murine experimental models, Bogduk
26) reported that each medial branch of the posterior spinal nerves innervate 2–3 lumbar facet joints. El-Mahdi et al.
27) also reported that symptomatic relief was obtained after sympathetic nerve block in patients with LBP caused by degenerative changes in the facet joint; and during the procedure, contrast media injected into the facet joint accumulated at the L1–2 nerve roots. Murata et al.
28) and Suseki et al.
29) described a relationship between the second lumbar spinal nerve and back pain. Hence, both irritation of the nerve root by FT at L2–3 and irritation of the medial branch of posterior spinal nerve can result in LBP.
The present study has several limitations. First, we are not sure whether it is feasible to look at FT as an isolated parameter; other degenerative changes such as disc degeneration, especially asymmetrical disc degeneration, leading to changes in coronal and sagittal balance may affect FT. In addition, the causal relationship between FT and LBP is unclear. Further research focusing on symptomatic relief after intra-articular injection to the facet joint may shed light on the causality. Second, the prevalence of FT and its association with LBP or dynamic LBP require further investigation. Additional studies on the pathological mechanism of FT are warranted. Furthermore, variance in the facet angle among individuals and the definition of asymmetry may remain controversial.
The prevalence of FT among community-based populations was 46.3%. Severe FT (a difference of ≥ 14°), most commonly affected the L4–5, with a prevalence of 24.7%. FT at L3–4, L4–5, and L5–S1 levels showed no correlation with LBP and dynamic LBP, but the relative risk of FT-associated LBP at L2–3 was approximately 1.6, and the relative risk of FT-associated dynamic LBP at L2–3 was approximately 1.7.
Go to :









 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download