Abstract
Appropriate pharmacologic therapy can reduce symptoms and risk and severity of exacerbations, as well as improve the health status and exercise tolerance of patients with chronic obstructive pulmonary disease. The most important medications for treating chronic obstructive pulmonary disease are inhaled bronchodilators including beta2-agonist and anticholinergics. Inhaled corticosteroids as anti-inflammatory drug should be considered in certain patients with caution considering risk and benefit. The choice within each class depends on the availability of medication and the patient's responses and preferences. Each treatment regimen needs to be individualized as the relationship between severity of symptoms, airflow limitation and severity of exacerbation can differ between patients.
Figures and Tables
 | Figure 1Classification of patients with chronic obstructive pulmonary disease. FEV1, forced expiratory volume in 1 second; mMRC, modified Medical Research Council dyspnea scale; CAT, chronic obstructive pulmonary disease assessment test. |
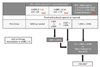 | Figure 2Pharmacologic treatment algorithms. FEV1, forced expiratory volume in 1 second; AE COPD, acute exacerbation of chronic obstructive pulmonary disease; mMRC, modified Medical Research Council dyspnea scale; CAT, COPD assessment test; SABA, short acting beta2-agonist; LAMA, long acting muscarinic antagonist; LABA, long acting beta2-agonist; ICS, inhaled corticosteroid; PDE4, phosphodiesterase 4. a)Postbronchodilator FEV1 <50%, symptoms of chronic bronchitis, and a history of exacerbations. b)Asthma overlap or high blood eosinophil. |
References
1. Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000; 320:1297–1303.

2. Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, Conway WA Jr, Enright PL, Kanner RE, O'Hara P. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994; 272:1497–1505.

3. Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 1999; 353:1819–1823.

4. Jenkins SC, Heaton RW, Fulton TJ, Moxham J. Comparison of domiciliary nebulized salbutamol and salbutamol from a metered-dose inhaler in stable chronic airflow limitation. Chest. 1987; 91:804–807.

5. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356:775–789.

6. Kew KM, Mavergames C, Walters JA. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; (10):CD010177.

7. Korn S, Kerwin E, Atis S, Amos C, Owen R, Lassen C. INSIST study group. Indacaterol once-daily provides superior efficacy to salmeterol twice-daily in COPD: a 12-week study. Respir Med. 2011; 105:719–726.

8. Dahl R, Chung KF, Buhl R, Magnussen H, Nonikov V, Jack D, Bleasdale P, Owen R, Higgins M, Kramer B. INVOLVE (INdacaterol: Value in COPD: Longer Term Validation of Efficacy and Safety) Study Investigators. INdacaterol: Value in COPD: Longer Term Validation of Efficacy and Safety) Study Investigators. Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax. 2010; 65:473–479.

9. Hanania NA, Feldman G, Zachgo W, Shim JJ, Crim C, Sanford L, Lettis S, Barnhart F, Haumann B. The efficacy and safety of the novel long-acting β2 agonist vilanterol in patients with COPD: a randomized placebo-controlled trial. Chest. 2012; 142:119–127.

10. Maleki-Yazdi MR, Beck E, Hamilton AL, Korducki L, Koker P, Fogarty C. A randomised, placebo-controlled, Phase II, dose-ranging trial of once-daily treatment with olodaterol, a novel long-acting β2-agonist, for 4 weeks in patients with chronic obstructive pulmonary disease. Respir Med. 2015; 109:596–605.

11. Decramer ML, Chapman KR, Dahl R, Frith P, Devouassoux G, Fritscher C, Cameron R, Shoaib M, Lawrence D, Young D, McBryan D. INVIGORATE investigators. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med. 2013; 1:524–533.

12. Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten-van Molken MP, Beeh KM, Rabe KF, Fabbri LM. POET-COPD Investigators. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011; 364:1093–1103.

13. Donohue JF, Fogarty C, Lotvall J, Mahler DA, Worth H, Yorgancioglu A, Iqbal A, Swales J, Owen R, Higgins M, Kramer B. INHANCE Study Investigators. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010; 182:155–162.

15. Casaburi R, Kukafka D, Cooper CB, Witek TJ Jr, Kesten S. Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest. 2005; 127:809–817.

16. Kesten S, Casaburi R, Kukafka D, Cooper CB. Improvement in self-reported exercise participation with the combination of tiotropium and rehabilitative exercise training in COPD patients. Int J Chron Obstruct Pulmon Dis. 2008; 3:127–136.

17. Cazzola M, Molimard M. The scientific rationale for combining long-acting beta2-agonists and muscarinic antagonists in COPD. Pulm Pharmacol Ther. 2010; 23:257–267.

18. Oba Y, Sarva ST, Dias S. Efficacy and safety of long-acting β-agonist/long-acting muscarinic antagonist combinations in COPD: a network meta-analysis. Thorax. 2016; 71:15–25.

19. Buhl R, Maltais F, Abrahams R, Bjermer L, Derom E, Ferguson G, Flezar M, Hebert J, McGarvey L, Pizzichini E, Reid J, Veale A, Gronke L, Hamilton A, Korducki L, Tetzlaff K, Waitere-Wijker S, Watz H, Bateman E. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). Eur Respir J. 2015; 45:969–979.

20. Wedzicha JA, Decramer M, Ficker JH, Niewoehner DE, Sandstrom T, Taylor AF, D'Andrea P, Arrasate C, Chen H, Banerji D. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013; 1:199–209.

21. Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT, Thach C, Fogel R, Patalano F, Vogelmeier CF. FLAME Investigators. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N Engl J Med. 2016; 374:2222–2234.

22. Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA. INSPIRE Investigators. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008; 177:19–26.

23. Zheng Y, Zhu J, Liu Y, Lai W, Lin C, Qiu K, Wu J, Yao W. Triple therapy in the management of chronic obstructive pulmonary disease: systematic review and meta-analysis. BMJ. 2018; 363:k4388.

24. Calzetta L, Cazzola M, Matera MG, Rogliani P. Adding a LAMA to ICS/LABA Therapy: a meta-analysis of triple combination therapy in COPD. Chest. 2019; 155:758–770.
25. Bafadhel M, Peterson S, De Blas MA, Calverley PM, Rennard SI, Richter K, Fageras M. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pul-monary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med. 2018; 6:117–126.

26. Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, Dransfield MT, Halpin DMG, Han MK, Jones CE, Kilbride S, Lange P, Lomas DA, Martinez FJ, Singh D, Tabberer M, Wise RA, Pascoe SJ. IMPACT Investigators. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018; 378:1671–1680.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download