Abstract
Purpose
To evaluate a new gonioscopy score and preoperative factors as a potential predictor for intraocular pressure (IOP) reduction after phacoemulsification.
Methods
This is a retrospective review of 182 eyes with glaucoma of either open or narrow angles that underwent phacoemulsification. Preoperative variables such as age, IOP, refractive errors, anterior chamber depth (ACD), axial length, and lens position were evaluated at 6 months after surgery. A preoperative gonioscopy score was created, summing the Shaffer gonioscopy grading in 4 quadrants. To determine variables associated with IOP change at 6 months, univariate and multivariate linear regression analysis was performed.
Results
The mean age of the patients was 72.8 ± 9.5 years and the average preoperative IOP was 16.4 ± 3.7 mmHg with 1.2 glaucoma medications. The mean IOP reduction after phacoemulsification was 2.7 ± 2.2 mmHg at postoperative 6 months. Preoperative IOP (β = 0.55, p < 0.001), gonioscopy score (β = −0.29, p < 0.001), ACD (β = −0.67, p = 0.02), and IOP/ACD ratio (β = 0.58, p = 0.01) were associated with IOP reduction at 6 months.
Conclusions
Preoperative predictors for IOP reduction after phacoemulsification were preoperative IOP, ACD, gonioscopy score, and IOP/ACD ratio in patients with glaucoma. The IOP/ACD ratio and gonioscopy score can be easy parameters to obtain and may help clinicians to estimate the IOP reduction after phacoemulsification.
Go to : 
References
1. Masis M, Mineault PJ, Phan E, Lin SC. The role of abdominal in glaucoma therapy: a systematic review and meta-analysis. Surv Ophthalmol. 2018; 63:700–10.
2. Slabaugh MA, Chen PP. The effect of cataract extraction on abdominal pressure. Curr Opin Ophthalmol. 2014; 25:122–6.
3. Issa SA, Pacheco J, Mahmood U, et al. A novel index for predicting intraocular pressure reduction following cataract surgery. Br J Ophthalmol. 2005; 89:543–6.

4. Bhallil S, Andalloussi IB, Chraibi F, et al. Changes in intraocular pressure after clear corneal phacoemulsification in normal patients. Oman J Ophthalmol. 2009; 2:111–3.

5. Huang G, Gonzalez E, Peng PH, et al. Anterior chamber depth, abdominal angle width, and intraocular pressure changes after abdominal: narrow vs open iridocorneal angles. Arch Ophthalmol. 2011; 129:1283–90.
6. Chen PP, Lin SC, Junk AK, et al. The effect of phacoemulsification on intraocular pressure in glaucoma patients: a report by the American Academy of Ophthalmology. Ophthalmology. 2015; 122:1294–307.
7. Guan H, Mick A, Porco T, Dolan BJ. Preoperative factors abdominal with IOP reduction after cataract surgery. Optom Vis Sci. 2013; 90:179–84.
8. Yang HS, Lee J, Choi S. Ocular biometric parameters associated with intraocular pressure reduction after cataract surgery in normal eyes. Am J Ophthalmol. 2013; 156:89–94.

9. Hsia YC, Moghimi S, Coh P, et al. Anterior segment parameters as predictors of intraocular pressure reduction after phacoemulsification in eyes with open-angle glaucoma. J Cataract Refract Surg. 2017; 43:879–85.

10. Lin SC, Masis M, Porco TC, Pasquale LR. Predictors of abdominal pressure after phacoemulsification in primary open-angle glaucoma eyes with wide versus narrower angles (an American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc. 2017; 115:T6.
11. Shaffer RN. A new classification of the glaucomas. Trans Am Ophthalmol Soc. 1960; 58:219–25.
12. Jahn CE. Reduced intraocular pressure after phacoemulsification and posterior chamber intraocular lens implantation. J Cataract Refract Surg. 1997; 23:1260–4.

13. Hayashi K, Hayashi H, Nakao F, Hayashi F. Influence of cataract surgery on automated perimetry in patients with glaucoma. Am J Ophthalmol. 2001; 132:41–6.

14. Huang G, Gonzalez E, Lee R, et al. Association of biometric abdominals with anterior chamber angle widening and intraocular pressure reduction after uneventful phacoemulsification for cataract. J Cataract Refract Surg. 2012; 38:108–16.
15. Lee RY, Huang G, Cui QN, et al. Association of lens vault with abdominal angles among different ethnic groups. Curr Eye Res. 2012; 37:486–91.
16. Park Y, Moon JI, Cho KJ. The effect of intraoperative factors on abdominal pressure reduction after phacoemulsification in open-abdominal glaucoma. J Korean Ophthalmol Soc. 2018; 59:930–7.
17. Dooley I, Charalampidou S, Malik A, et al. Changes in intraocular pressure and anterior segment morphometry after uneventful abdominal cataract surgery. Eye (Lond). 2010; 24:519–27.
18. Wang N, Chintala SK, Fini ME, Schuman JS. Ultrasound activates the tm elam-1/il-1/nf-κ b response: a potential mechanism for abdominal pressure reduction after phacoemulsification. Invest Ophthalmol Vis Sci. 2003; 44:1977–81.
19. Zhao Z, Zhu X, He W, et al. Schlemm's canal expansion after abdominal phacoemulsification surgery: an optical coherence abdominal study. Invest Ophthalmol Vis Sci. 2016; 57:6507–12.
20. Alvarado JA, Katz LJ, Trivedi S, Shifera AS. Monocyte abdominal of aqueous outflow and recruitment to the trabecular abdominal following selective laser trabeculoplasty. Arch Ophthalmol. 2010; 128:731–7.
Go to : 
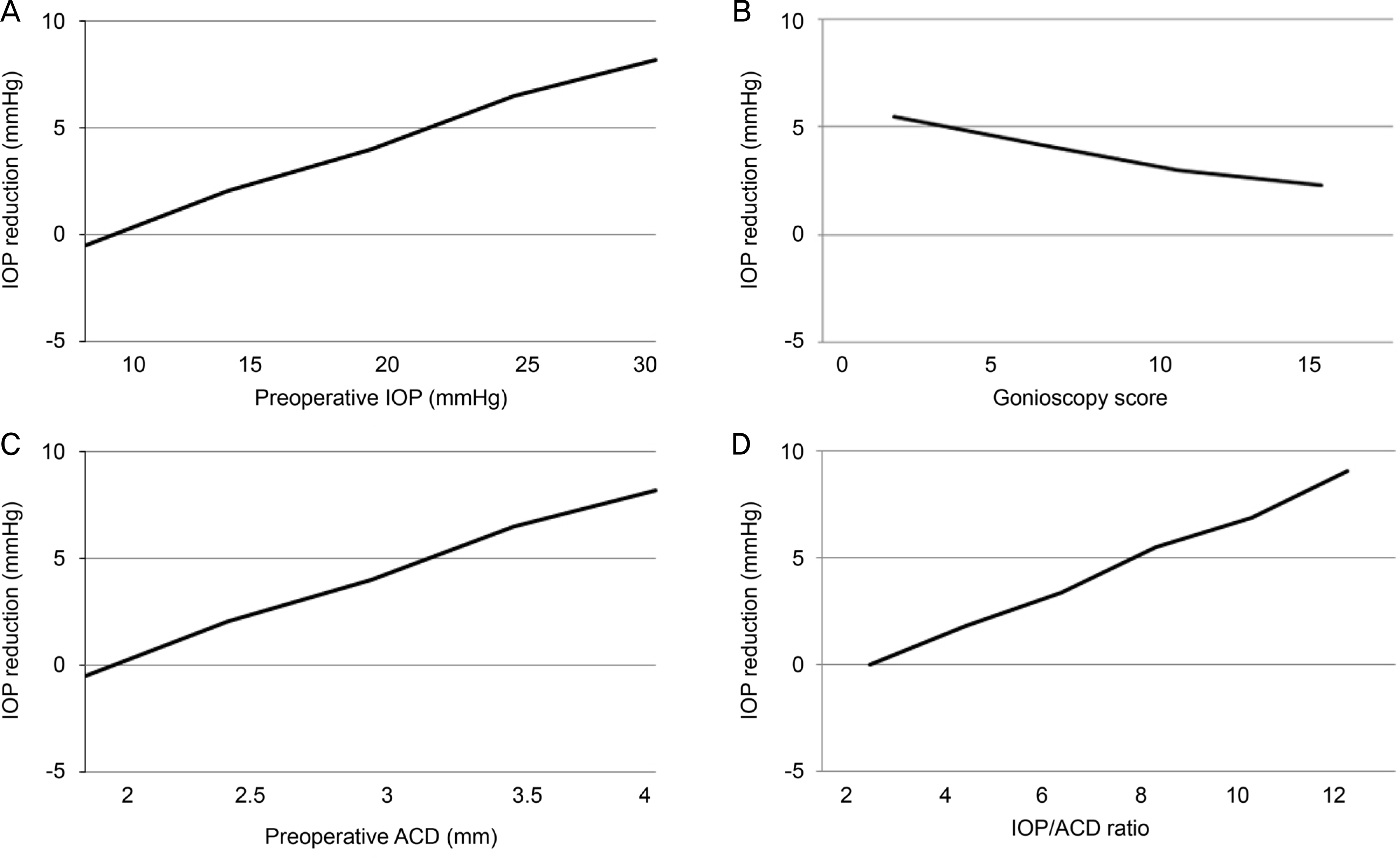 | Figure 1.The relationship between significant preoperative predictors with IOP reduction following phacoemulsification after multivariate analysis. (A) Relationship between preoperative IOP and IOP reduction. (B) Relationship between gonioscopy score and IOP reduction. (C) Relationship between preoperative ACD and IOP reduction. (D) Relationship between IOP/ACD ratio and IOP reduction are presented. IOP = intraocular pressure; ACD = anterior chamber depth. |
Table 1.
Baseline demographic and biometric characteristics of the patients
Table 2.
Univariate and multivariate analysis of the association between clinical preoperative predictors and changes in IOP at 6 months after phacoemulsification
| Parameter |
Univariate analysis |
Multivariate analysis* |
||
|---|---|---|---|---|
| β | p-value | β | p-value | |
| Age (years) | 0.03 | 0.39 | – | – |
| Sex (female/male) | 0.02 | 0.64 | – | – |
| Preoperative IOP (mmHg) | 0.63 | <0.001 | 0.55 | <0.001 |
| Gonioscopy score (n) | −0.31 | <0.001 | −0.29 | <0.001 |
| ACD (mm) | −0.75 | 0.01 | −0.67 | 0.02 |
| Axial length (mm) | −0.28 | 0.02 | −0.21 | 0.07 |
| CCT (μ m) | 0.01 | 0.53 | – | – |
| Preoperative spherical equivalent (D) | 0.09 | 0.48 | – | – |
| Lens thickness (mm) | 0.12 | 0.12 | – | – |
| IOP/ACD ratio | 0.89 | <0.001 | 0.58 | 0.01 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download