Abstract
Objectives
The purpose of this study was to investigate the combined effects of Galla chinensis extract (GCE) and calcium (CA) on enamel remineralization. The antibacterial effect of G. chinensis on Streptococcus mutans biofilm was also evaluated by examining the bacterial growth, acidogenesis, and morphology of the biofilm in vitro.
Methods
S. mutans biofilm was formed on bovine enamel specimens over a 72-h period and treated for 10 min with 1.0 mol CA, 4,000 ppm aqueous solution of GCE, or a combination of the two (GCE+CA). The enamel specimens were analyzed for enamel surface microhardness after remineralization. We tested the anti-cariogenic effects of GCE based on the inhibition of acid production, antibacterial activity, and morphological changes in S. mutans. The differences between the groups and antibacterial effects were analyzed using one-way analysis of variance.
Results
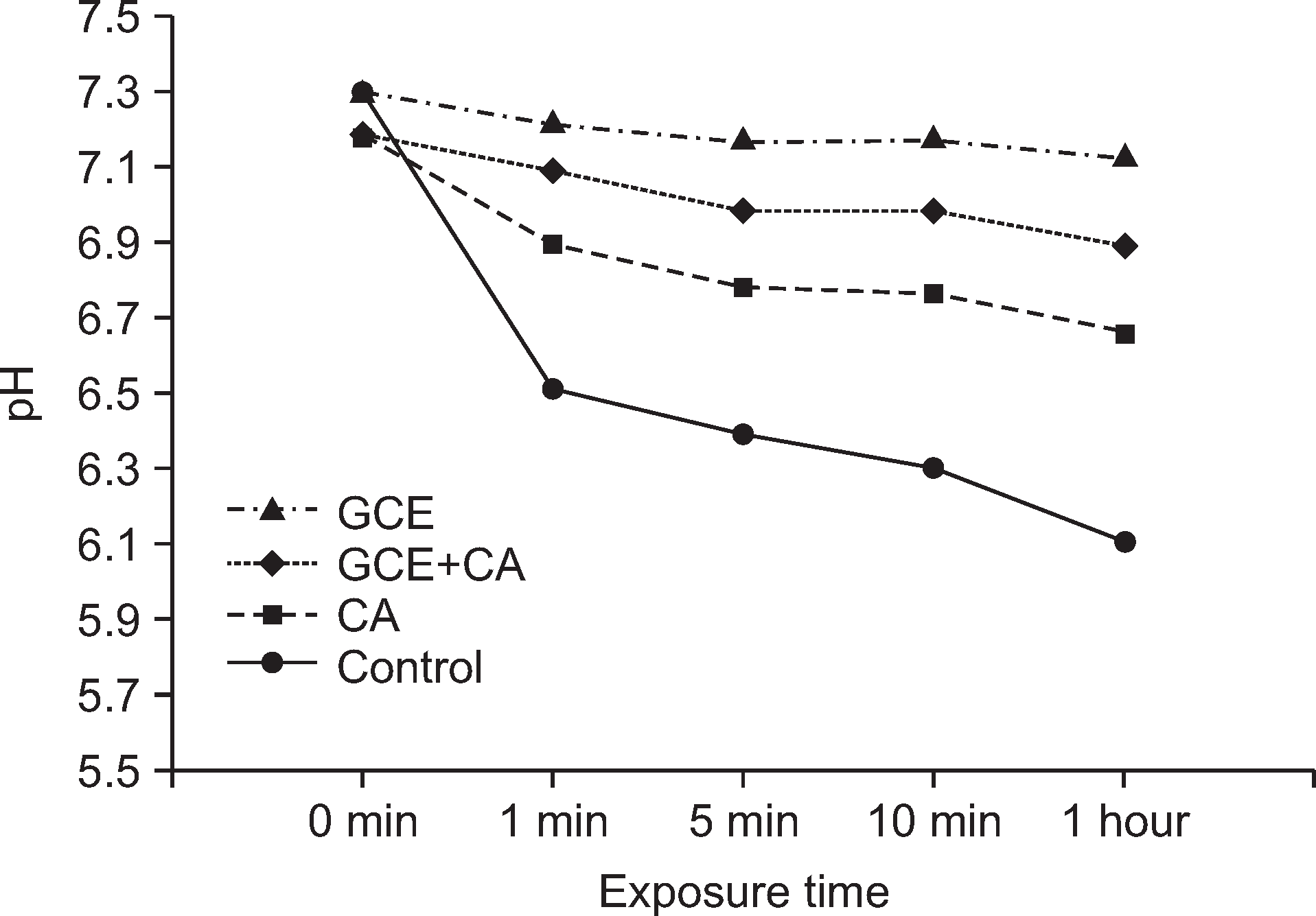
GCE+CA group showed the highest efficacy in enhancing remineralization. The GCE group showed the highest antibacterial activity against S. mutans biofilm. Although the GCE+CA group showed significant antibacterial activity, it was less than that of the GCE group (P<0.05). Both GCE and GCE+CA groups maintained a pH of approximately 7.0 for 1 h whereas the pH of the control group decreased rapidly from pH 7.3 to pH 6.1. SEM imaging revealed that S. mutans treated with GCE and GCE+CA showed irregular cell wall structure and showed fewer cells in the chain than the typical long chains observed in the control group.
Go to : 
References
1. Cheng L, Li JY, Huang S, Zhou XD. Effect of Galla Chinensis on enhancing remineralization of enamel crystals. Biomed Mater. 2009; 4:1–6.
2. Zero DT. Dental caries process. Dent Clin North Am. 1999; 43:635–664.
3. Phan TN, Marquis RE. Triclosan inhibition of membrane enzymes and glycolysis of Streptococcus mutans in suspensions and biofilms. Can J Microbiol. 2006; 52:977–983.

4. Jeon J, Osalen P, Falsetta M, Koo H. Natural products in caries research: current (limited) knowledge, challenges and future perspective. Caries Res. 2011; 45:243–263.

5. Palombo EA. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral disease. Evid Based Complement Alternat Med. 2011; 1–15.
6. Huang Z, Zhou X, Li J, Liu T, Li H, Zhu B. The effects of traditional Chineses medicines on the adherence of Streptococcus mutans to salivary acquired pellicle in vitro. Sichuan Da Xue Xue Bao Yi Xue Ban. 2003; 34:135–137.
7. Xie Q, Li JY, Zuo YL, Zhou XD. The effect of Galla Chinensis on the growth of cariogenic bacteria in vitro. Hua Xi Kou Qiang Yi Xue Za Zhi. 2005; 23:82–84.
8. Cheng L, ten Cate JM. Effect of Galla Chinensis on the in vitro remineralization of advanced enamel lesions. Int J Oral Sci. 2010; 2:15–20.

9. Liu Z, Liu T, Li J, Zhou X, Zhang J. The effect of Galla Chinensis on the demineralization of enamel. Sichuan Da Xue Xue Bao Yi Xue Ban. 2003; 34:507–509.
10. Lei C, Jiyao L, Yuquing H, Xuedong Z. Effect of compounds of Galla Chinensis and their combined effects with fluride on remineralization of initial enamel lesion in vitro. J Dent. 2008; 36:369–373.
11. Chu JP, Li JY, Hao YQ, Zhou XD. Effect of compounds of Galla Chinensis on remineralisation of initial enamel carious lesion in vitro. J Dent. 2007; 35:383–387.
12. Koo H, Hayacibara MF, Schobel BD, Cury JA, Rosalen PL, Park YK, et al. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimi-crob Chemother. 2003; 52:782–789.

13. Koo HI, Pearson SK, Scott-Anne K, Abranches J, Cury JA, Rosalen PL, et al. Effects of apigenin and tt-farnesol on glucosyltransferase activity, biofilm viability and caries development in rats. Oral Mi-crobiol Immunol. 2002; 17:337–343.

14. Koo H, Seils J, Abranches J, Burne RA, Bowen WH, Quivey RG. Influence of apigenin on gtf gene expression in Streptococcus mutans UA159. Antimicrob Agents Chemother. 2006; 50:542–546.
15. Flötra L, Gjermo PER, Rölla G, Waerhaug J. Side effects of chlorhexidine mouth washes. Scand J Dent Res. 1971; 79:119–125.
16. Flötra L. Different modes of chlorhexidine application and related local side effects. J Periodontal Res. 1973; 8:41–44.

17. Xie Q, Li J, Zhou X. Anticaries effect of compounds extracted from Galla Chinensis in a multispecies biofilm model. Oral Microbiol Im-munol. 2008; 23:459–465.
18. Grobler SR, van der Horst G. Biochemical analysis of various cool drinks with regard to enamel erosion, de- and remineralization. J Dent Assoc S Afr. 1982; 37:681–684.
19. Li Y, Tang R. Study of effects of Chinese nutgall on normal enamel and non-organic enamel demineralization. J Clin Stomatol. 2006; 22:344–346.
20. Lee YS, Han OK, Bae MJ, Kim KJ, Shin SW, Lee SK, et al. Antimicrobial and anticancer effects of Galla Rhois on pathogens isolated from oral and KB human oral epidermoid carcinoma cells. Korean J Oriental Physiology & Pathology. 2003; 17:1427–1432.
21. Curzon MEJ, Hefferren JJ. Modern methods for assessing the cariogenic and erosive potential of foods. Br Dent J. 2001; 191:41–46.

22. Chu JP, Li JY, Hao YQ, Zhou XD. Effect of compounds of Galla Chinensis on remineralisation of initial enamel carious lesion in vitro. J Dent. 2007; 35:383–387.
23. Kang MS, Oh JS, Kang IC, Hong SJ, Choi CH. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J Microbiol. 2008; 46:744–750.

24. Tian F, Li B, Ji B, Yang J, Zhang G, Chen Y, et al. Antioxidant and antimicrobial activities of consecutive extracts from Galla Chinensis : The polarity affects the bioactivities. Food Chem. 2009; 113:173–179.
25. Kim JE, Kim HE, Hwang JK, Lee HJ, Kwon HK, Kim BI. Antibacterial characteristics of Curcuma xanthorrhiza extract on Streptococcus mutans biofilm. J Microbiol. 2008; 46:228–232.

26. Wu-Yuan CD, Chen CY, Wu RT. Gallotannins inhibit growth, water-insoluble glucan synthesis, and aggregation of mutans streptococci. J Dent Res. 1998; 67:51–55.

27. Camargo CH, Bernardineli N, Valera MC, de Carvalho CA, de Oliveira LD, Menezes MM, et al. Vehicle influence on calcium hydroxide pastes diffusion in human and bovine teeth. Dent Trauma-tol. 2006; 22:302–306.

28. Yassen GH, Platt JA, Hara AT. Bovine teeth as substitute for human teeth in dental research: a review of literature. J Oral Sci. 2011; 53:273–282.

29. Huang S, Gao S, Cheng L, Yu H. Combined effects of nano-hydroxyapatite and Galla Chinensis on remineralization of initial enamel lesion in vitro. J Dent. 2010; 38:811–819.
Go to : 
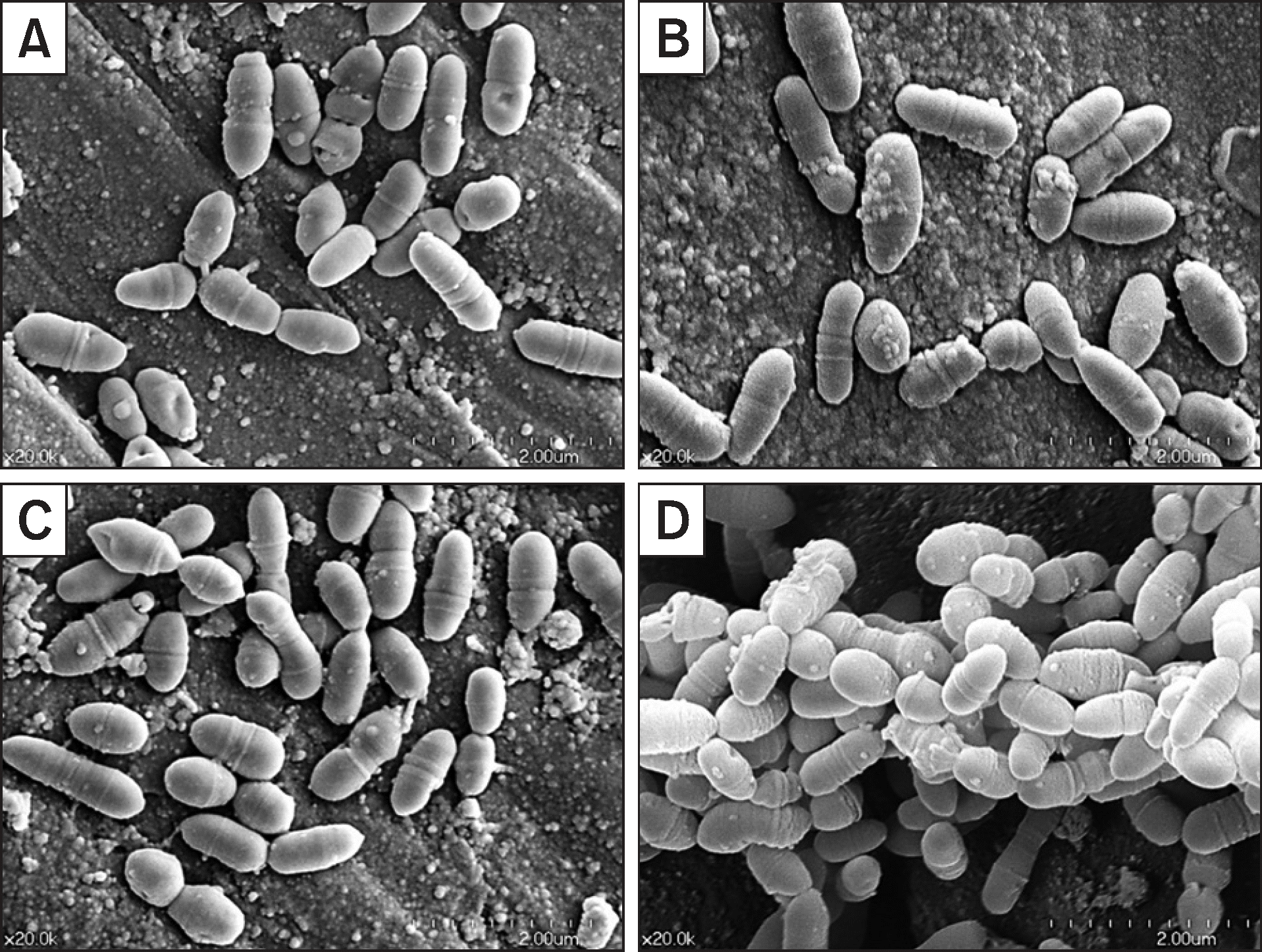 | Fig. 3.SEM images of the S. mutans biofilm after 10 min treatment. (A) GCE, (B) GCE+CA, (C) CA, and (D) Control. |
Table 1.
Comparison of the surface microhardness of different groups after remineralization
| Condition | N |
VHN |
VHN |
DVHN |
P |
|---|---|---|---|---|---|
| Baseline | After artificial caries formation | After remineralization | |||
| GCE | 24 | 308.8±5.1 | 48.70±5.00 | 41.8±3.9a | 0.009* |
| GCE+ CA | 24 | 308.9±6.3 | 46.85±4.95 | 44.2±3.9a | |
| CA | 24 | 303.2±6.3 | 42.72±5.19 | 27.1±5.4b | |
| Control | 12 | 307.3±7.3 | 44.85±4.85 | 0.5±3.9c |
Values are mean±SD. DVHN = After remineralization VHN - Before treatment VHN (baseline). CA, immersed in 1.0 mol CaCl2 for 10 min; GCE, immersed in 4,000 ppm GCE for 10 min; CA+GCE, immersed in 1.0 mol CaCl2 and 4,000 ppm GCE for 10 min; Control, no treatment. *Statistically significant by repeated measured ANOVA at the a=0.05 level.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download