Abstract
Guillain-Barré syndrome (GBS) and acute disseminated encephalomyelitis (ADEM) are demyelinating neurologic disorders with different target organs. Although they share similar pathogenetic mechanism, reports of simultaneous occurrence of the 2 disorders are rare. A 2 year 6 month old girl visited our hospital for fever, cough, and general weakness. Although the muscle power of extremities showed mild weakness and voiding difficulty, initial deep tendon reflex of both knees and ankles was normal. A nerve conduction study to evaluate the weakness revealed the absence of F waves. Cerebrospinal fluid analysis demonstrated pleocytosis with lymphocyte predominance and elevated protein levels. Magnetic resonance imaging showed abnormal T2 hyperintensity in pons, medulla and spinal cord. Serum anti-GD1b antibody was positive. Based on clinical findings, laboratory findings, nerve conduction study, and neuroimaging, the diagnosis of GBS and ADEM was made. This is the first case of GBS accompanied by ADEM in Korea.
Guillain-Barré syndrome (GBS) and acute disseminated encephalomyelitis (ADEM) are caused by immunologic dysregulation commonly associated with a prior infection or vaccination.12 GBS produces inflammatory demyelination of the peripheral nervous system (PNS). Characteristic features of GBS are progressive, symmetric muscle weakness with decreased deep tendon reflex.12 ADEM is a demyelinating disorder of the central nervous system (CNS) and encephalopathy is the cardinal feature.3 Pathogenesis of GBS and ADEM is not completely understood. Despite the difference of targeted neurologic system, these two disorders are commonly triggered by environmental stimuli and explained as immune responses against the nervous system.123 Co-occurrence of GBS and ADEM is uncommon. We report a Korean girl with GBS concurrent with ADEM.
A 2 year 6 month old girl presented with general weakness for 2 days. She had fever and cough for 11 days. Her oral intake decreased to 10% of usual meals, the sleep duration was longer than usual, and she demonstrated voiding difficulty. At the time of admission, the patient was drowsy presenting a Glasgow Coma Scale (GCS) of 14/15 (eye 3, motor 6, verbal 5) with the following vital sign: blood pressure, 100/60 mmHg; pulse, 156/min; and body temperature, 37.4℃. Upon examination, coarse breathing sound with rale was heard on both lung fields. Neurologically, there was no cranial nerve dysfunction, and her motor power in the upper and lower extremities was decreased to grade IV and III respectively with no sensory abnormalities. Deep tendon reflexes were intact with negative Babinski sign. Ataxia, ophthalmoplegia, and facial palsy were not observed.
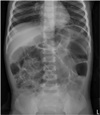
White blood cell count (20,190/uL, segmented neutrophil 26%) and C-reactive protein level (1.28 mg/dL) increased. Metabolic studies and thyroid function tests were normal. Lumbar puncture revealed pleocytosis (99 cells/high power field; 82% lymphocytes) with elevated protein (77.3 mg/dL) and negative gram stain and culture. Polymerasechain reactions for several respiratory virus and mycoplasma pneumonia antibodies were all negative. Chest x-ray showed peribronchial infiltration in the left lower lobe, and abdomen x-ray showed paralytic ileus due to the autonomic disturbances (Fig. 1).
Cerebrospinal fluid (CSF) analysis showed pleocytosis mainly lymphocyte, so intravenous delivery of mannitol was initiated under suspicion of aseptic meningoencephalitis. In addition, intravenous ampicillin/sulbactam was started for pneumonia.
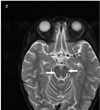
On third hospital day, her knee reflexes were decreased to +/+, and ankle reflexes were absent. She showed stuporous mentality. Nerve conduction studies (NCSs) indicated an early stage acute inflammatory demyelinating polyradiculoneruopathy with absent F waves in a nerve conduction study. The motor and sensory conduction velocities were normal. She was given intravenous immunoglobulin (400 mg/kg/day) for 5 days upon a diagnosis of GBS. Intrathecal immunoglobulin synthesis and oligoclonal bands were not detected. Anti-GD1b antibody immunoglobulin M was detected with high titer 55.61 (normal: < 20) and anti-GQ1b antibody immunoglobulin G was negative. Electroencephalography was normal during sleep, Brain magnetic resonance imaging (MRI) showed diffuse and subtle T2 hyperintensity in both pons and medulla. Spine MRI revealed nonspecific T2 hyperintensity at conus medullaris of spinal cord (Fig. 2). She was diagnosed with GBS based on weakness and loss of deep tendon reflex of extremities, abnormal nerve conduction study, monophasic course, anti-GD1b. Concurrent ADEM was diagnosed based on her encephalopathy, negative findings in viral serology and bacterial culture of the CSF, an abnormal brain imaging showing T2 hyperintensity in brainstem, and exclusion of other causes for encephalopathy.
Although the present patient had encephalopathy and abnormal findings in brainstem on brain MRI, we excluded Bickerstaff's brainstem encephalopathy and Miller-Fisher syndrome because there was no typical symptom such as ataxia and ophthalmoplegia and anti-GQ1b antibody was negative. Cases of ADEM with demyelinating lesions confined to the brainstem have been reported.4
She was given intravenous immunoglobulin (400 mg/kg/day) for 5 days. A follow-up brain MRI and spinal MRI performed 4 weeks after the end of treatment were normal (Fig. 3). Knee jerks in both sides and motor weakness have fully resolved and she has been doing well for over 6 months.
The international Pediatric Multiple Sclerosis Study Group (IPNSSG) provided criteria of ADEM, including 1) a first clinical CNS event with presumed inflammatory demyelinating case, 2) encephalopathy, not explained by fever, 3) no new clinical and MRI finding emerge 3 months or more after the onset, and 4) abnormal brain MRI during acute phase.4 GBS can be diagnosed when there is bilateral weakness of limbs, decreased deep tendon reflexes, monophasic course, albumino-cytologic dissociation, consistent NCS findings, and absence of alternative diagnosis for weakness.5 Several potential mechanisms have been suggested, which include immune responses to a common epitope component of both PNS and CNS myelin and general susceptibility to autoimmune disease.678 Coincidental occurrence might be possible. However, these do not explain the precise mechanisms of co-occurrence and the rarity of simultaneous occurrence of peripheral and central demyelinating disorders.
Our patient had simultaneous GBS and ADEM. PNS involvement was proven by the clinical and electrophysiological studies, CSF findings, and positive serum anti-GD1b antibody. CNS involvement was identified by brain MRI, spinal MRI, and pleocytosis in CSF. Although acute inflammatory demyelinating disorders of the nervous system selectively involve either CNS or PNS, demyelinating diseases of both systems are related to autoimmune mechanisms.123 ADEM usually affects CNS, and GBS predominantly involves PNS, but few studies present involvement of others. Considering acute onset of disease, presenting encephalopathy, no history of neurologic signs suggestive of earlier demyelinating episode, and T2 hyperintensity in Brain MRI, the patient diagnosed with ADEM. Through the onset of acute motor sign, absent reflexes, and lumbar puncture revealing albumino-cytologic dissociation, the diagnosis of GBS was made. In our case, the patient showed positive serum anti-GD1b antibody closely associated with GBS, which means not a spectrum of ADEM involves PNS.
To our knowledge, there have been only four
reports of seven patients with concomitant ADEM
and GBS in children.6789 Bernard, et al reported a case of GBS and ADEM which evolved to chronic
inflammatory demyelinating polyradiculoneuropathy.8 GBS and ADEM occurred simultaneously or sequentially
in all patients. The range of age was from 2.6 to 16 years. Our patient was 2.6 years old, and is the youngest patient reported thus far. 5 patients among 7 patients had acute infectious illness, but there was no correlation with vaccination. Peripheral demyelinating neuropathy was demonstrated by electrophysiological studies, such as electromyography and NCS. Central demyelinating lesions were identified by CSF findings and neuroimaging. All patients were treated with methylprednisone or intravenous immunoglobulin. Prognosis was very good in all patients. Pleocytosis of CSF was observed in 4 out of 7 patients with GBS and ADEM in children. Our patient also showed CSF pleocytosis. The CSF cell count in GBS is typically normal and pleocytosis is an unusual finding of GBS. In clinical GBS, elevated cell counts alert the physician to consider alternative diagnosis.10 Infectious myelitis caused by enterovirus, herpes simplex virus, Epstein-Barr virus, and cytomegalovirus can show similar clinical symptoms with GBS.10 Human immunodeficiency virus infection associated with GBS can produce CSF pleocytosis,11 and CNS demyelination associated with GBS can present with CSF pleocytosis.678910
In summary, we report a girl with GBS associated with ADEM. Although simultaneous GBS and ADEM are rare, the combination of the two diseases has been continuously reported. Further study is needed to explain this combination and its prognosis.
Figures and Tables
References
1. Jones HR Jr. Guillain-Barré syndrome: Perspectives with infants and children. Semin Pediatr Neurol. 2000; 7:91–102.

2. van Doorn PA. Diagnosis, treatment and prognosis of Guillain-barré syndrome (GBS). Presse Med. 2013; 42:e193–e201.

3. Leake JA, Albani S, Kao AS, Senac MO, Billman GF, Nespeca MP, et al. Acute disseminated encephalomyelitis in childhood: Epidemiologic, clinical and laboratory features. Pediatr Infect Dis J. 2004; 23:756–764.

4. Tenembaum S, Chitnis T, Ness J, Hahn JS. International Pediatric MS Study Group. Acute disseminated encephalomyelitis. Neurology. 2007; 68:S23–S36.

5. Fokke C, van den Berg B, Drenthen J, Walgaard C, van Doorn PA, Jacobs BC. Diagnosis of Guillain-Barré syndrome and validation of brighton criteria. Brain. 2014; 137:33–43.

6. Okumura A, Ushida H, Maruyama K, Itomi K, Ishiguro Y, Takahashi M, et al. Guillain- Barré syndrome associated with central nervous system lesions. Arch Dis Child. 2002; 86:304–306.
7. Amit R, Shapira Y, Blank A, Aker M. Acute, severe, central and peripheral nervous system combined demyelination. Pediatr Neurol. 1986; 2:47–50.

8. Bernard G, Riou E, Rosenblatt B, Dilenge ME, Poulin C. Simultaneous Guillain-Barré syndrome and acute disseminated encephalomyelitis in the pediatric population. J Child Neurol. 2008; 23:752–757.

9. Mohammed RR, Jan MM. Co-morbid Guillainbarré syndrome and acute disseminated encephalomyelitis. Neurosciences (Riyadh). 2013; 18:166–168.
10. Rauschka H, Jellinger K, Lassmann H, Braier F, Schmidbauer M. Guillain–Barré syndrome with marked pleocytosis or a significant proportion of polymorphonuclear granulocytes in the cerebrospinal fluid: Neuropathological investigation of five cases and review of differential diagnoses. Eur J Neurol. 2003; 10:479–486.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download