Abstract
Objectives
We designed the study to compare the oncologic and renal function outcomes of off-clamp, laparoscopic partial nephrectomy (OCLPN) and conventional laparoscopic partial nephrectomy (HCLPN) for renal tumors.
Methods
Between March 2008 and July 2015, 114 patients who underwent laparoscopic partial nephrectomy (LPN) of a renal neoplasm were studied. We performed LPN without hilar clamp on 40 patients (OCLPN, Group 1), and conventional LPN with hilar control and renorrhaphy on another 40 patients (HCLPN, Group 2). We retrospectively reviewed the medical records of each patient's age, sex, R.E.N.A.L. nephrometry score (RNS), operation time, complications, hospitalization period, tumor size, positive resection margin, histologic classification of tumor, pathologic stage, Fuhrman grade, estimated blood loss (EBL), warm ischemic time (WIT), and estimated glomerular filtration rate (eGFR) before and one year after surgery.
Results
There were no significant differences in age, sex, preoperative eGFR, EBL, surgical (anesthesia) time, and tumor size between the two groups. The mean eGFR was not significantly different between the OCLPN and HCLPN groups 1 month (95 and 86.2 mL/min/1.73 m2, respectively; P = 0.106), 6 months (92.9 and 83.6 mL/min/1.73 m2, respectively; P = 0.151) and 12 months (93.8 and 84.7 mL/min/1.73 m2, respectively; P = 0.077) postoperatively. The change in eGFR after one year was 3.9% in the OCLPN group and −7.9% in the HCLPN group.
Partial nephrectomy (PN) has been shown to be equivalent to radical nephrectomy (RN) in T1b neoplasms as well as clinical T1a neoplasms, and is superior in terms of preservation of renal function.12345 A number of studies have reported no difference in oncologic outcome between laparoscopic partial nephrectomy (LPN) and open partial nephrectomy (OPN).678
In OPN, cold ischemia using ice is typically used to reduce renal ischemic injury. However, in laparoscopy, warm ischemia is generally used because of the difficulty of cold ischemia.91011 In general, limiting warm ischemic time (WIT) to 30 minutes is considered to be sufficient for preserving renal function. In some studies using renal scintigraphy, the time limit of WIT for minimizing renal function is 25 minutes.121314
Many surgeons use bulldog clamps to perform renal artery ligation in LPN. Vessel loops (Rummel tourniquets) and laparoscopic Satinsky clamps are also used. In addition, there have been reports of selective ligation of the feeding artery or ligation of the branchial renal artery. In the case of a protruding tumor in the renal upper pole or lower pole, a method of direct compression of the renal parenchyma without ligation of the blood vessel using Simon renal pole clamps has been reported.151617
Renal artery ligation may cause renal ischemia and reperfusion injury, and progression to third stage chronic renal disease has been reported in up to 8–30% of cases. In cases of chronic renal disease, progression to end-stage renal disease is likely.18192021 In order to reduce the possibility of such permanent renal function decrease without ligation of the renal artery, selective clamping of renal artery branches is performed when partial resection of the renal neoplasm is attempted. However, this procedure is limited because of its difficulty. Nevertheless, because of the absence of renal ischemia and reperfusion, it is expected to be beneficial and has recently been performed by a small number of surgeons.2223
The authors have retrospectively reviewed changes in renal function and oncologic results after off-clamp, laparoscopic partial nephrectomy (OCLPN) and conventional hilar clamp, laparoscopic partial nephrectomy (HCLPN) for at least one year in renal neoplasm patients.
Of the 114 patients who underwent LPN of new renal tumors from March 2008 to July 2015, 80 patients who had no diabetes or hypertension at the time of diagnosis, and who had medical records and followed up for at least 12 months were selected for the study. 40 patients underwent OCLPN, and 40 patients underwent HCLPN.
We retrospectively reviewed medical records such as patient history, operative records, biopsy results, and outpatient records. Specifically, we reviewed medical records of each patient's age, sex, R.E.N.A.L. nephrometry score (RNS), operation time, complications (modified Clavien-Dindo classification), hospitalization period, tumor size, positive resection margin, histologic classification of tumor, pathologic stage, Fuhrman grade, estimated blood loss (EBL), warm ischemic time (WIT), and estimated glomerular filtration rate (eGFR) before and one year after surgery.
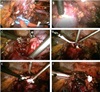
All surgeries were performed in a conventional manner by a single surgeon (T.S. Kim) (Fig. 1).2425 OCLPNs used a peritoneal approach under general anesthesia. A bulldog clamp or vessel loop was placed at the renal artery to prepare for excessive bleeding (Fig. 1A). The renal veins were not always clamping. A monopolar hook electrode was used before the resection of the neoplasms to mark the resection plane with a dot or line (Fig. 1B). Tumors were resected using a 10-mm cold knife to minimize loss of normal parenchyma (Fig. 1C). Floseal® (Baxter Healthcare Corporation, Deerfield, IL, USA) or Tissel™ (Baxter Healthcare Corporation) was applied to the resected surface after resection of the tumor (Fig. 1D). Surgicel® (Ethicon, a Johnson & Johnson company, Cincinnati, OH, USA) and Gelfoam® (Pharmacia & Upjohn Co., a division of Pfizer, New York, NY, USA) were used to fill the defect and then sutured using V-locTM (Fig. 1E). The kidney parenchyma, peripheral fat, and Gerota's fascia were sutured using 3–0 vicryl® (Ethicon, a Johnson & Johnson company, Cincinnati, OH, USA) (Fig. 1F). HCLPN was performed in the same manner as OCLPN except that the renal artery was clamped with a bulldog clamp or a vessel loop.
Follow-up was performed at the 1st, 6th, and 12th months postoperatively. A physical examination, blood test, urinalysis, and chest radiograph were included. All patients underwent abdominal computed tomography at the 6th and 12th months.Renal function was compared using eGFR calculated by the Modification of Diet in Renal Disease formula.
All statistics were analyzed using PASW version 18.0 (SPSS Inc., Chicago, IL, USA).The clinical characteristics of the two groups were assessed using Chi-square test for dependent variables and Student's t-test for continuous variables. A p-value less than 0.05 was considered statistically significant.
There were no significant differences between the OCLPN and HCLPN groups in age (mean 55.1 vs 58.8 years), gender (M: F, 6: 4), preoperative eGFR (mean 90.3 vs 92 mL/min/1.73 m2) and percentage of patients with low (4−6) RNS (75% vs 70%) (Table 1).
Similarly, no significant differences were found in EBL (mean 329 vs 319.5 mL), operation (anesthesia) time (mean 179.5 vs 180.6 min), hospitalization (mean 8.9 days vs 9.1 days) and tumor size (mean 2.9 cm vs 3.0 cm).
Within the HCLPN group, the WIT was 23.3 min on average. A complication of Clavien-Dindo classification III or more was reported in one HCLPN patient. The patient underwent open radical nephrectomy two days after surgery due to delayed bleeding. Five OCLPN cases and one HCLPN case were margin-positive. Of these, three OCLPN cases and the HCLPN case were benign tumors. The preoperative eGFR was 90.3 mL/min/1.73m2 in OCLPN cases and 92 mL/min/1.73 m2 in HCLPN cases. The mean eGFR was not significantly different between the OCLPN and HCLPN groups 1 month (95 vs 86.2 mL/min/1.73 m2, P = 0.106), 6 months (92.9 vs 83.6 mL/min/1.73 m2, P = 0.151) and 12 months (93.8 vs 84.7 mL/min/1.73 m2, P = 0.077) postoperatively, but the residual renal function remained high in the OCLPN group (Fig. 2). The one-year eGFR change was 3.9% in OCLPN and −7.9% in HCLPN.
In this study, there was no difference in operative factors (EBL, admission duration, etc.) between the two groups (OCLPN vs. HCLPN) with the same patient characteristics before surgery. After one year, renal function was superior in the OCLPN group.
EBL was expected to be greater in OCLPN than HCLPN at the time of surgery because of the lack of renal artery clamping, but no difference was found between the two groups. However, Smith et al. reported greater EBL in patients without renal artery clamping (mean 500 vs 200 mL).26 Bleeding is more likely to occur in laparotomy than in laparoscopic surgery because of the possibility of continuing the operation boldly under the operative field, due to the direct compression of the renal parenchyma, even in case of massive bleeding.
Wang et al. reported that bleeding was more frequent in laparoscopic OCLPN cases. However, this study was limited to 44 cases with low RNS and tumor size of 3 cm or less, and may not apply more generally.27 In contrast, other studies have shown that EBL is equivalent in OCLPN and HCLPN, with EBL depending on the surgeon's surgical technique, surgical expertise, and location of neoplasms.28 In this study, there was no difference in EBL between the two groups, since most of them were low RNS (75 vs 70%), and the risk of excessive bleeding during surgery was low and could be managed well.
In our study, the mean WIT in the HCLPN group was 23.3 min. There was a 7.9% decrease in eGFR at one-year follow-up in the HCLPN group, but there was no significant decrease in eGFR in the OCLPN group (+3.9%). This is because the ischemia-reperfusion injury is minimized by not ligating the blood vessels and supplying sufficient fluid before and after the operation. Bagheri et al. suggested that minimal resection of normal renal parenchyma is more important than ischemia-reperfusion injury associated with WIT.29 In our study, 75% of OCLPN cases and 70% of HCLPN cases had low RNS. It is thought that there was almost no decrease in renal function due to the relatively small amount of normal parenchyma in neoplasms with high RNS exophytic scores.In the meta-analysis of Trehan, OCLPN was found to be beneficial to renal function with a standardized weighted mean difference (SWMD) of 0.27.30 However, Shah et al. observed eGFR of patients with OCLPN (209 patients) and HCLPN (106 patients) up to 5 years postoperatively, and found no difference in eGFR between the two groups after 6 months.28 They attributed this to compensation of the opposite kidney and recovery of renal function of the diseased kidney. OCLPN is a good surgical procedure for low-RNS patients who are already suffering from decreased renal function, diabetes and hypertension and are expected to have decreased renal function in the future.
It is important to obtain a clear resection margin for better tumor treatment results when resecting a renal tumor. However, in OCLPN, it is difficult to obtain a resection margin precisely because the operation is performed during the continuation of hemorrhage. In this study, 12.5% (five cases) of the resected specimens had positive resection marginsin OCLPN patients. This rate is higher than that in HCLPN patients (2.5%; one case), which is higher than results of previous studies.30 However, three out of the five OCLPN cases with positive resection margins were renal angiomyolipomas; during surgery, the surgeon judged them to be lipomas and intentionally resected minimal renal parenchyma. Two of the OCLPN cases (6.25%) with positive resection margins were malignant tumors, similar to other studies.30 One of these patients recurred postoperatively and underwent radical nephrectomy. The HCLPN patient with positive surgical margins has had no recurrence to date. One patient who had negative surgical margins had recurrence and underwent radical nephrectomy.
This study is a retrospective study and has limitations in terms of the small number of patients.In addition, most of the patients were low RNS, and there was no significant difference in difficulty between the two methods, so EBL and surgical results may be comparable. Renal scintigraphy and other tests of the unilateral renal function could be used to better understand the effects of surgery on renal function, but were not performed on all patients and so were excluded from the analysis. In the future, we should investigate the effect of OCLPN on renal function, tumor treatment results, etc.
The purpose of this study was to determine the effects of renal artery clamping on renal function and oncologic outcomes in patients undergoing LPN. We analyzed patients with renal tumors undergoing LPN between 2008 and 2015. Renal function was measured using eGFR over a 12-month period. Although there were no statistically significant differences in eGFR between the groups from preoperative to 12-months postoperative, the eGFR change after 12 months was superior in the OCLPN group (OCLPN + 3.9% vs. HCLPN − 7.9%). Also, in the OCLPN group, the percentage of patients with positive resection margins was higher than in the HCLPN group. However, there was no significant difference between the two groups when comparing only malignant tumors.
OCLPN is superior to HCLPN in terms of functional outcome, with no significant difference in oncologic outcome. Therefore, we think that OCLPN is a good operative method for patients with low clinical stage who are worried about renal function decrease, diabetes or hypertension.
Figures and Tables
Fig. 1
Procedure of OCLPN
(A) Self-made Rummel tourniquets or bulldog clamps were hung on the renal hilar vessels
for quick vessel clamping. (B) Linear cauterization marking made by monopolar hook
electrode. (C) Marked margin around renal mass was excised using 10-mm Metzenbaum
scissors with minimal safety margins. (D) Biologic hemostatics such as Floseal and Tissel
were used in the excised renal bed. (E) Parenchymal defect was filled with Surgicel and
Gelfoam (F) Renal parenchyma, perirenal fat and Gerota's fascia were approximated using
V-loc and 3–0 Vicryl
References
1. Herr HW. Partial nephrectomy for unilateral renal carcinoma and a normal contralateral kidney : 10-year followup. J Urol. 1999; 161:33–34.
2. Lee CT, Katz J, Shi W, Thaler HT, Reuter VE, Russo P. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol. 2000; 163:730–736.

3. Simmons MN, Weight CJ, Gill IS. Laparoscopic radical versus partial nephrectomy for tumors >4 cm: intermediate-term oncologic and functional outcomes. Urology. 2009; 73:1077–1082.

4. Becker F, Siemer S, Hack M, Humke U, Ziegler M, Stöckle M. Excellent long-term cancer control with elective nephron-sparing surgery for selected renal cell carcinomas measuring more than 4 cm. Eur Urol. 2006; 49:1058–1063.
5. Leibovich BC, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004; 171:1066–1070.

6. Porpiglia F, Volpe A, Billia M, Scarpa RM. Laparoscopic versus open partial nephrectomy : analysis of the current literature. Eur Urol. 2008; 53:732–742.
7. Allaf ME, Bhayani SB, Rogers C, Varkarakis I, Link RE, Inagaki T, et al. Laparoscopic partial nephrectomy: evaluation of long-term oncological outcome. J Urol. 2004; 172:871–873.

8. Seifman BD, Hollenbeck BK, Wolf JS Jr. Laparoscopic nephron-sparing surgery for a renal mass: 1-year minimum follow-up. J Endourol. 2004; 18:783–786.

9. Gill IS, Abreu SC, Desai MM, Steinberg AP, Ramani AP, Ng C, et al. Laparoscopic ice slush renal hypothermia for partial nephrectomy: the initial experience. J Urol. 2003; 170:52–56.

10. Landman J, Venkatesh R, Lee D, Vanlangendonck R, Morissey K, Andriole GL, et al. Renal hypothermia achieved by retrograde endoscopic cold saline perfusion: technique and initial clinical application. Urology. 2003; 61:1023–1025.

11. Janetschek G, Abdelmaksoud A, Bagheri F, Al-Zahrani H, Leeb K, Gschwendtner M. Laparoscopic partial nephrectomy in cold ischemia: renal artery perfusion. J Urol. 2004; 171:68–71.

12. Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, et al. Renal function after partial nephrectomy: effect of warm ischemia relative to quantity and quality of preserved kidney. Urology. 2012; 79:356–360.

13. Funahashi Y, Hattori R, Yamamoto T, Sassa N, Fujita T, Gotoh M. Effect of warm ischemia on renal function during partial nephrectomy: assessment with new 99mTc -mercaptoacetyltriglycine scintigraphy parameter. Urology. 2012; 79:160–164.

14. Porpiglia F, Fiori C, Bertolo R, Angusti T, Piccoli GB, Podio V, et al. The effects of warm ischaemia time on renal function after laparoscopic partial nephrectomy in patients with normal contralateral kidney. World J Urol. 2012; 30:257–263.

15. Simon J, Meilinger M, Lang H, Hautmann RE, de Petriconi R. Novel technique for in situ cold perfusion in laparoscopic partial nephrectomy. Surg Endosc. 2008; 22:2184–2189.

16. Simon J, Bartsch G Jr, Finter F, Hautmann R, de Petriconi R. Laparoscopic partial nephrectomy with selective control of the renal parenchyma: initial experience with a novel laparoscopic clamp. BJU Int. 2009; 103:805–808.

17. Shao P, Qin C, Yin C, Meng X, Ju X, Li J, et al. Laparoscopic partial nephrectomy with segmental renal artery clamping: technique and clinical outcomes. Eur Urol. 2011; 59:849–855.

18. Shikanov S, Lifshitz D, Chan AA, Okhunov Z, Ordonez MA, Wheat JC, et al. Impact of ischemia on renal function after laparoscopic partial nephrectomy: a multicenter study. J Urol. 2010; 183:1714–1718.

19. Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, et al. Renal function after partial nephrectomy: effect of warm ischemia relative to quantity and quality of preserved kidney. Urology. 2012; 79:356–360.

20. Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, et al. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol. 2010; 58:340–345.

21. Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, et al. Comparison of warm ischemia versus no ischemia during partial nephrectomy on a solitary kidney. Eur Urol. 2010; 58:331–336.

22. Gill IS, Eisenberg MS, Aron M, Berger A, Ukimura O, Patil MB, et al. "Zero ischemia" partial nephrectomy: novel laparoscopic and robotic technique. Eur Urol. 2011; 59:128–134.

23. D'Urso L, Simone G, Rosso R, Collura D, Castelli E, Giacobbe A, et al. Benefits and shortcomings of superselective transarterial embolization of renal tumours before zero ischemia laparoscopic partial nephrectomy. Eur J Surg Oncol. 2014; 40:1731–1737.
24. Kang SH, Rhew HY, Kim TS. Changes in renal function after laparoscopic partial nephrectomy: comparison with laparoscopic radical nephrectomy. Korean J Urol. 2013; 54:22–25.

25. Kim TS, Oh JH, Rhew HY. "Off-clamp, non-renorrhaphy" laparoscopic partial nephrectomy with perirenal fat and Gerota's fascia reapproximation: initial experience and perioperative outcomes. J Laparoendosc Adv Surg Tech A. 2014; 24:339–344.

26. Smith GL, Kenney PA, Lee Y, Libertino JA. Non-clamped partial nephrectomy: techniques and surgical outcomes. BJU Int. 2011; 107:1054–1058.

27. Wang HK, Qin XJ, Ma CG, Shi GH, Zhang HL, Ye DW. Nephrometry score-guided off-clamp laparoscopic partial nephrectomy: patient selection and short-time functional results. World J Surg Oncol. 2016; 14:163.

28. Shah PH, George AK, Moreira DM, Alom M, Okhunov Z, Salami S, et al. To clamp or not to clamp? Long-term functional outcomes for elective off-clamp laparoscopic partial nephrectomy. BJU Int. 2016; 117:293–299.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download