Abstract
Objectives
Approximately 30% of individuals diagnosed with schizophrenia suffer from treatment-resistant schizophrenia. Clozapine is underutilized in the management of treatment-resistant schizophrenia. To understand contributing factors, we analyzed the time course and causes of clozapine discontinuations that occurred over a 20-year period in a clinical setting.
Methods
The reasons for discontinuation and duration of clozapine treatment from a retrospective database of 138 patients with schizophrenia who had prescribed clozapine at least a month were reviewed, with the motives for discontinuation coded. The causes for termination were analyzed.
Results
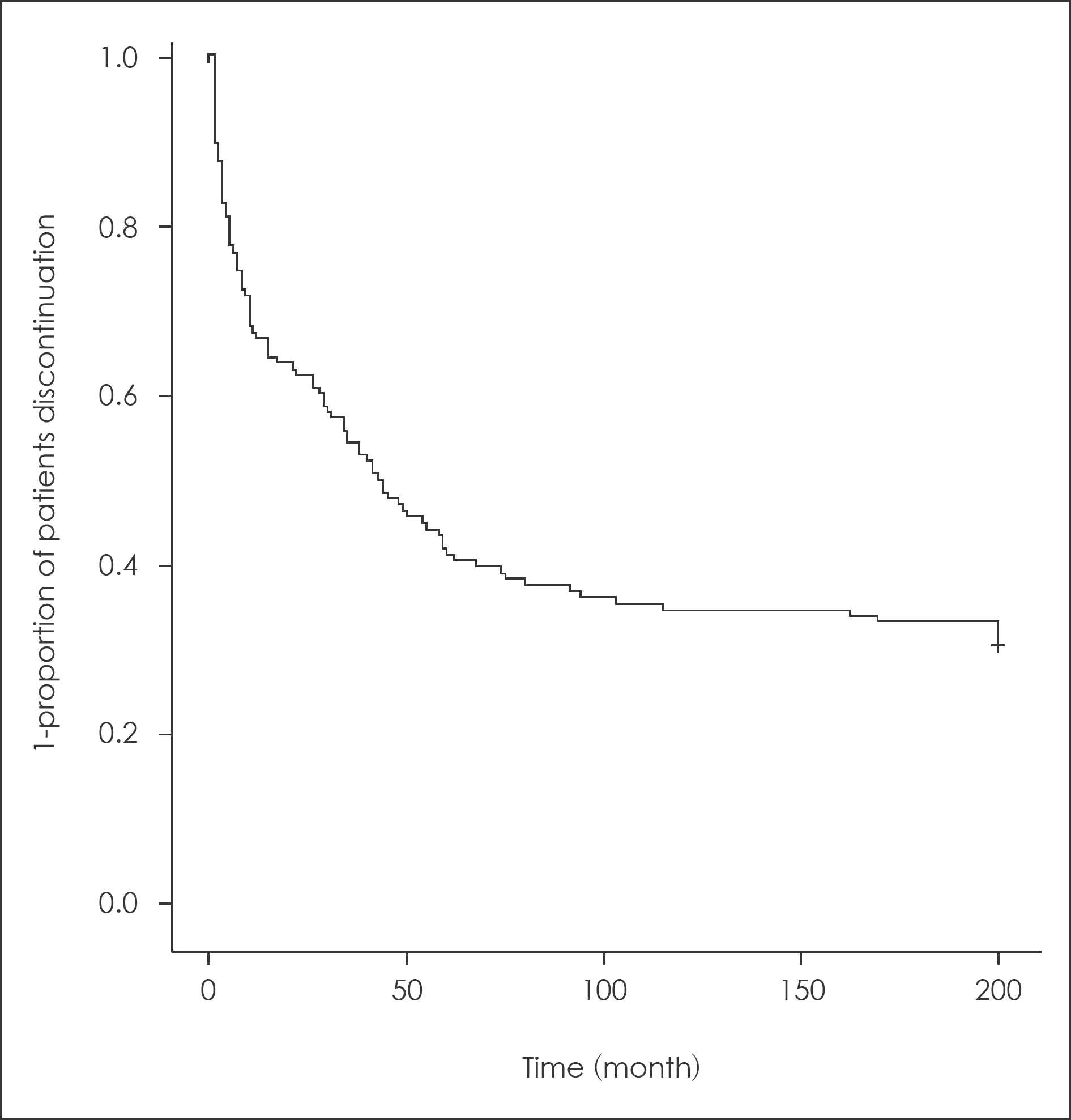
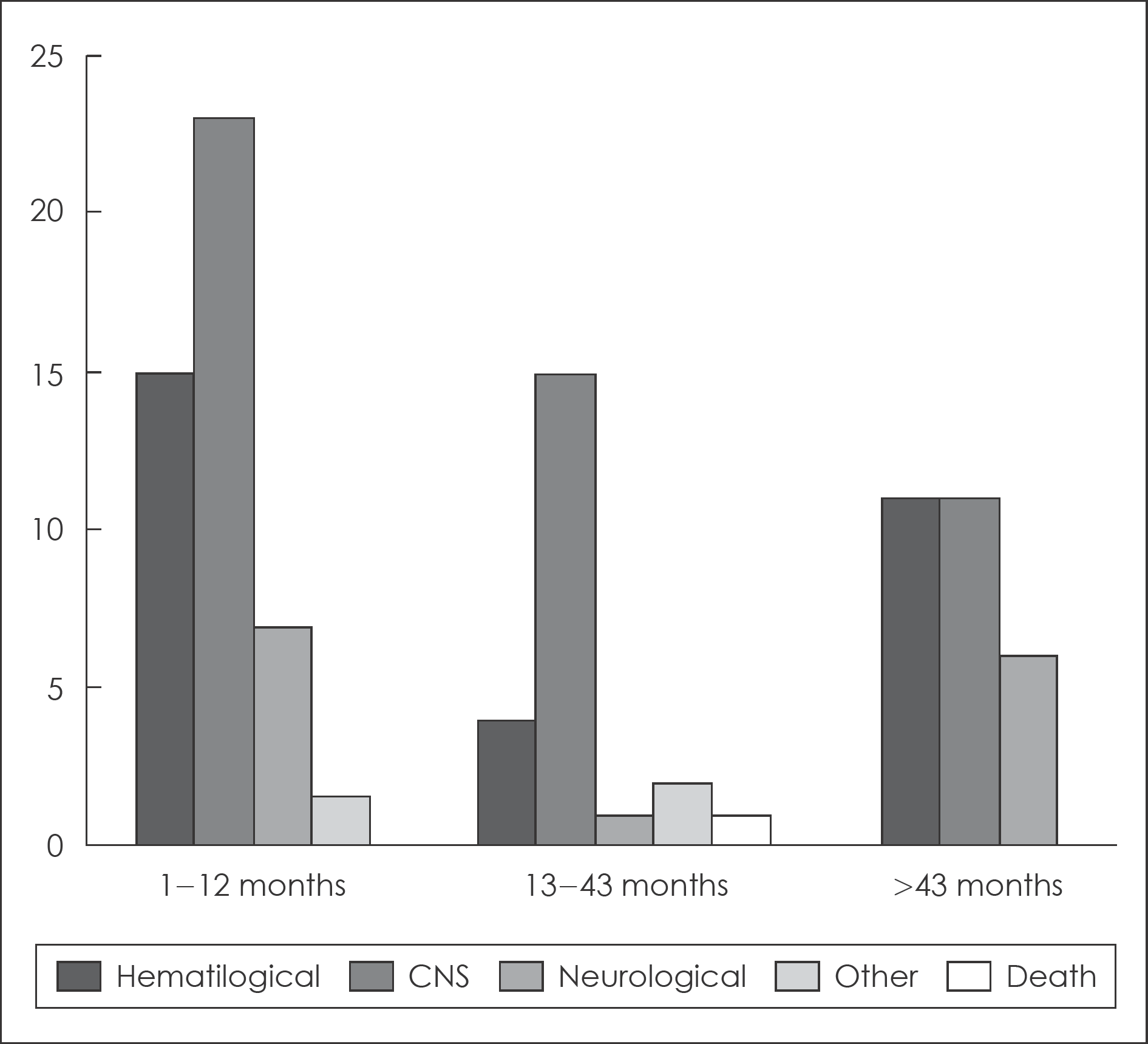
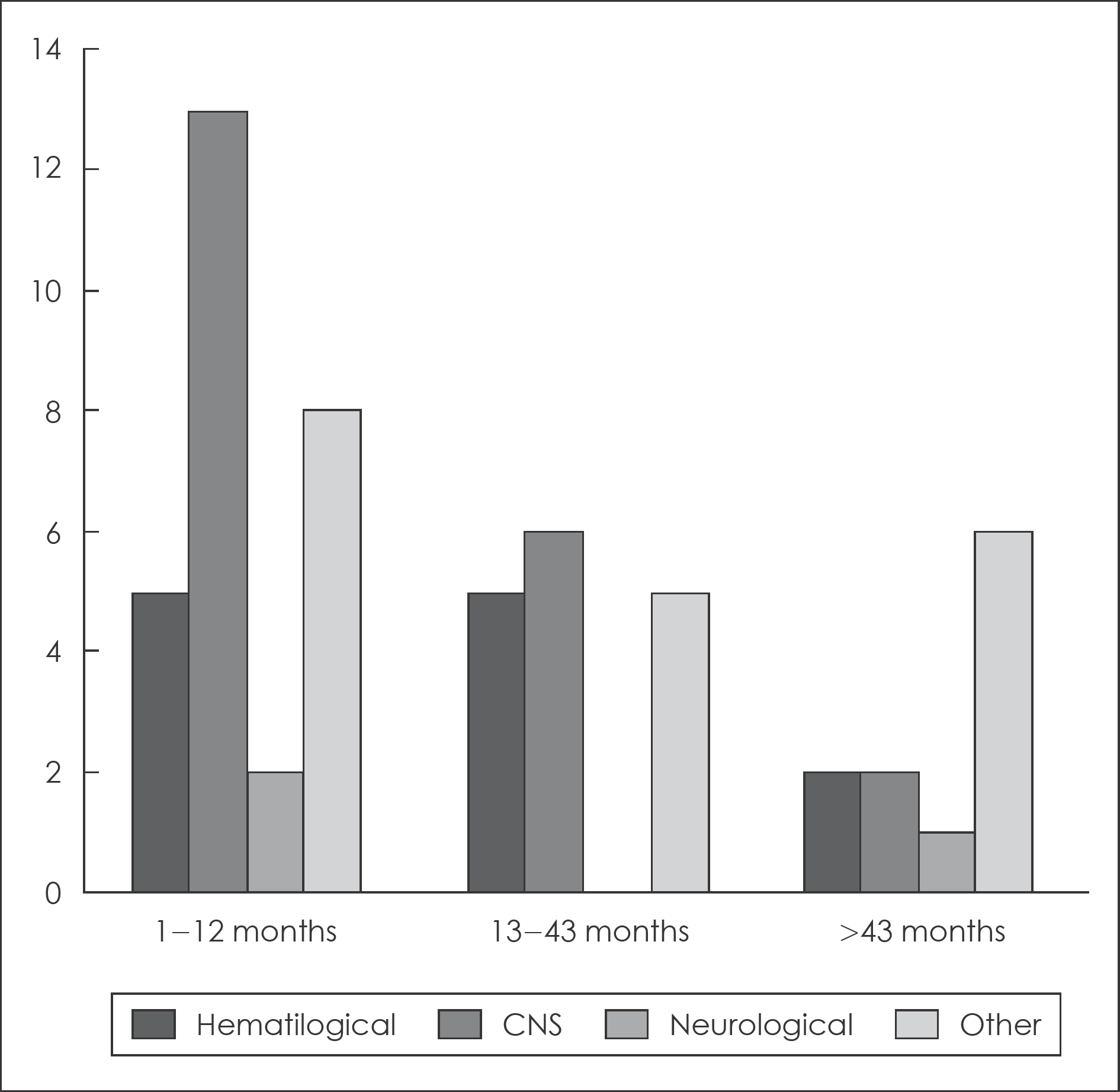
Over two-thirds of the patient had ceased clozapine. The two most common causes for discontinuation were side-ef-fects (50%), and own decision (30%). Somnolence accounted for 34% of all side-effects induced discontinuations. Hematologi-cal problems accounted for 23% of side-effect. The Maximal treatment dose of clozapine was higher in continuation group (442.36 mg) than in discontinuation group (397.26 mg). The CGI-S score when prescribing clozapine last was higher in discontinuation group than in continuous group. The patients who took atypical antipsychotics before clozapine tended to cease clozapine because of side-effects than who took typical agent.
Go to : 
REFERENCES
1). Levine SZ, Rabinowitz J, Faries D, Lawson AH, Scher-Svanum H. Treatment response trajectories and antipsychotic medications: examination of up to 18 months of treatment in the CATIE chronic schizophrenia trial. Schizophr Res. 2012; 137:141–146.
2). Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988; 45:789–796.
3). Meltzer HY, Bartani B, Kwon KY, Ramirez LF, Burnett S, Sharpe J. A prospective study of clozapine in treatment-resistant schizophrenia patients. I. Preliminary report. Psychopharmacology. 1989; 99:S68–S72.
4). Glick ID, Correll CU, Altamura AC, Marder SR, Csemansky JG, Weiden PJ, et al. Mid-term and longterm efficacy and effectiveness of antipsychotic medications for schizophrenia: a data-driv-en, personalized clinical approach. J Clin Psychiatry. 2011; 72:1616–1627.
5). Lewis SW, Barnes TR, Davies L, Murray RM, Dunn G, Hayhurst KP, et al. Randomized controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull. 2006; 32:715–723.

6). McEvoy JP, Lieberman JA, Stroup TS, Davis SM, Meltzer HY, Rosenheck RA, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006; 163:600–610.

7). Lieberman JA, Kane JM, Johns CA. Clozapine: Guidelines for clonical management. J Clin Psychiatry. 1989; 50:329–338.
8). Wheeler A, Humberstone V, Robinson G. Outcomes for schizophrenia patients with clozapine treatment: how good does it get? J Psychopharmacol. 2009; 23:957–965.

10). Henneen J, Baldessarini R. Suicidal risk during treatment with clozapine: a metaanalysis. Schizophr Res. 2005; 73:139–145.
11). Howes OD, Vergunst F, Gee S, McGuire P, Kapur S, Taylor D. Adherence to treatment guidelines in clinical practice: study of anti-psychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012; 201:481–485.

12). Nielsen J, Damkier P, Lublin H, Taylor D. Optimizing clozapine treatment. Acta Psychiatr Scand. 2011; 123:411–422.

13). Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. 2012; 6:134–144.
14). Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005; 19(Suppl 1):1–93.
15). Tuunainen A, Wahlbeck K, Gilbody SM. Newer atypical antipsychotic medication versus clozapine for schizophrenia. Cochrane Database Syst Rev. 2000. CD000966.

16). Fitzsimons J, Berk M, Lambert T, Bourin M, Dodd S. A review of clozapine safety. Expert Opin Drug Saf. 2005; 4:731–744.

17). Davis MC, Fuller MA, Strauss ME, Konicki PE, Jaskiw GE. Discontinuation of clozapine: a 15-year naturalistic retrospective study of 320 patients. Acta Psychiatr Scand. 2014; 130:30–39.

18). Patel M, de Zoysa N, David A. Cross-sectional study of patients per-spectives on adherence to antipsychotic medication: depot versus oral. J Clin Psychiatry. 2008; 69:1548–1556.
19). Zygmunt A, Olfson M, Boyer C, Mechanic D. Interventions to im-prove medication adherence in schizophrenia. Am J Psychiatry. 2002; 159:1653–1654.

20). Taylor D, Douglas-Hall P, Olofinjana B, Whiskey E, Thomas A. Reasons for discontinuing clozapine: matched, case-control comparison with risperidone long-lasting injection. Br J Psychiatry. 2009; 94:165–167.
21). Munro J, O'Sullivan D, Andrews C, Arana A, Mortimer A, Ker-win R. Active monitoring of 12,760 clozapine recipients in the UK and Ireland. Br J Psychiatry. 1999; 175:576–580.

22). Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005; 353:1209–1223.

23). Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Davis JM. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2012; 5:CD008016.

24). Kahn RS, Fleischhacker WW, Boter H, Davidson M, Verqouwe Y, Keet IP, et al. Effectiveness of antipsychotic drugs in firstepisode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008; 371:1085–1097.

25). Szymanski SR, Cannon TD, Gallacher F, Erwin RJ, Gur RE. Course of treatment response in firstepisode and chronic schizophrenia. Am J Psychiatry. 1996; 153:519–525.
26). Ciudad A, Haro JM, Alonso J, Bousono M, Suarez D, Novick D, et al. The Schizophrenia Outpatient Health Outcomes (SOHO) study: 3-year results of antipsychotic treatment discontinuation and related clinical factors in Spain. Eur Psychiatry. 2008; 23:1–7.

27). Taylor M, Shajahan P, Lawrie SM. Comparing the use and discontinuation of antipsychotics in clinical practice: an observation-al study. J Clin Psychiatry. 2008; 69:240–245.
28). Hodgson R, Belgamwar R, Al Tawarah Y, Mackenzie G. The use of atypical antipsychotics in the treatment of schizophrenia in North Staffordshire. Hum Psychopharmacol. 2005; 20:141–147.

29). Haro JM, Novick D, Belger M, Jones PB. Antipsychotic type and correlates of antipsychotic treatment discontinuation in the outpatient treatment of schizophrenia. Eur Psychiatry. 2006; 21:41–47.

30). Ciapparelli A, Dell'Osso L, Bandettini PA, Carmassi C, Cecconi D, Fenzi M, et al. Clozapine in treatment-resistant patients with schizophrenia, schizoaffective disorder, or psychotic bipolar disorder: a naturalistic 48-month followup study. J Clin Psychiatry. 2003; 64:451–458.
31). Laker MK, Duffett RS, Cookson JC. Longterm outcome with clozapine: comparison of patients continuing and discontinuing treatment. Int Clin Psychopharmacol. 1998; 13:75–78.
32). Seabourne A, Thomas CS. The use of clozapine in South Man-chester. Psychiatric Bull. 1994; 18:618–619.

33). Krivoy A, Malka L, Fischel T, Weizman A, Valevski A. Predictors of clozapine discontinuation in patients with schizophrenia. Int Clin Psychopharmacol. 2011; 26:311–315.

34). Atkinson JM, Douglas-Hall P, Fischetti C, Sparshatt A, Taylor DM. Outcome following clozapine discontinuation: a retrospective analysis. J Clin Psychiatry. 2007; 68:1027–1030.
35). Fenton WS, Blyler CR, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997; 23:637–651.

36). Hudson TJ, Owen RR, Thrush CR, Han X, Pyne JM, Thapa P, et al. A pilot study of barriers to medication adherence in schizophrenia. J Clin Psychiatry. 2004; 65:211–216.

37). Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988; 45:789–796.
38). Young C, Bowers M, Mazure C. Management of the adverse effects of clozapine. Schizophr Bull. 1998; 24:381–390.

39). Longden E, Read J. Assessing and reporting the adverse effects of antipsychotic medication: A Systematic Review of clinical studies, and prospective retrospective, and cross-sectional research. Clin Neuropharmacol. 2016; 39:29–39.
40). Scheepers FE, de Wied CC, Hulshoff Pol HE, van de Flier W, van der Linden JA, Kahn RS. The effect of clozapine on caudate nucle-us volume in schizophrenic patients previously treated with typical antipsychotics. Neuropsychopharmacol. 2001; 24:47–54.

41). Leung JG, Chengappa KN, Ivanov E, Gandotra G, Kahn CE, Weber JS, et al. Antipsychotic agents used to augment clozapine during longterm inpatient hospitalizations. Pharmacopsychiatry. 2014; 47:263–267.

Go to : 
Table 1.
Factors for clozapine discontinuation
Table 2.
Sociodemographic and clinical characteristics of the subjects
| Total | Continuation, n (%) | Discontinuation, n (%) | χ2 | p-value | |
|---|---|---|---|---|---|
| Whole Sample | 137 | 41 (30.4) | 96 (69.6) | ||
| Gender | |||||
| Male | 73 | 23 (56.1) | 50 (52.1) | 0.186 | 0.666 |
| Female | 64 | 19 (43.9) | 46 (47.9) | ||
| Education | |||||
| 0-12 | 75 | 24 (64.9) | 51 (60.7) | 0.188 | 0.665 |
| >12 | 47 | 13 (35.1) | 33 (39.3) | ||
| Married | |||||
| Single | 102 | 30 (73.2) | 72 (76.6) | 0.528 | 0.768 |
| Married | 25 | 9 (22.0) | 16 (17.0) | ||
| Divorced | 8 | 2 (4.9) | 6 (6.4) | ||
| Living arrangement | |||||
| Living alone | 17 | 3 (7.3) | 14 (15.1) | 1.538 | 0.215 |
| Living with family | 117 | 39 (92.7) | 79 (84.9) | ||
| Job | |||||
| No | 86 | 23 (57.5) | 63 (66.3) | 0.946 | 0.331 |
| Yes∗ | 49 | 17 (42.5) | 32 (33.7) | ||
| Economic status | |||||
| Low | 37 | 10 (25.6) | 27 (30.0) | 0.253 | 0.615 |
| High | 92 | 29 (74.4) | 63 (70.0) | ||
| Concomitant antipsychoti | ics | ||||
| No | 74 | 13 (31.7) | 61 (63.5) | 11.722 | 0.001 |
| Yes | 63 | 28 (68.3) | 35 (36.5) |
Table 3.
Comparison between continuation group and discontinuation group
| Continuation, Mean (SD) | Discontinuation, Mean (SD) | t | p | |
|---|---|---|---|---|
| Age of onset | 21.13 (7.53) | 22.80 (8.04) | -1.124 | 0.263 |
| Age at clozapine start | 29.85 (12.52) | 29.52 (10.74) | 0.158 | 0.875 |
| Duration of untreatment | 10.19 (32.26) | 9.79 (19.48) | 0.082 | 0.935 |
| Hospitalization number before using clozapine | 2.93 (3.28) | 2.13 (2.54) | 1.377 | 0.174 |
| Hospitalization number in the use of clozapine | 0.63 (1.75) | 0.50 (0.88) | 0.553 | 0.581 |
| Body mass index (BMI) | 23.37 (3.01) | 24.15 (4.56) | -1.054 | 0.295 |
| Clozapine maximal dose∗ | 442.36 (90.40) | 397.26 (95.34) | 2.362 | 0.020 |
| Clozapine last prescription dose∗ | 279.29 (137.00) | 260.27 (127.06) | 0.710 | 0.480 |
| Clinical Global Impression-Severity (CGI-S) scale before starting clozapine | 5.71 (1.12) | 5.75 (1.18) | -0.201 | 0.842 |
| Clinical Global Impression-Severity (CGI-S) scale when prescribing clozapine last | 3.12 (0.78) | 3.93 (1.06) | -4.356 | <0.001 |
Table 4.
Clinical characteristics of the subjects




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download