This article has been corrected. See "Erratum: Endoscopic Features of Upper Gastrointestinal Tract in Patients with Systemic Sclerosis Compared to the Healthy Control" in Volume 26 on page 147.
Abstract
Objective
To characterize the endoscopic features of upper gastrointestinal tract in patients with systemic sclerosis (SSc) compared with those in the healthy controls.
Methods
Data on esophagogastroduodenoscopy (EGD) in 180 patients with SSc (SSc group) were compared with that from the 181 age- and sex-matched healthy control who underwent EGD for routine checkup (control group). Clinical data of participants at the time of EGD (defined as baseline) were collected from electric medical record. Endoscopic findings were evaluated by two experts with blinded to their clinical features. Primary outcome of the study was prevalence of each endoscopic lesion between the two groups.
Results
The mean±standard deviation age and disease duration in the SSc group at baseline were 55.3±11.8 and 2.9±3.7 years, respectively. Compared to the control group, SSc group more frequently showed reflux esophagitis (32.8% vs. 9.4%, p<0.001). In contrast, prevalence of atrophic gastritis was significantly lower in the SSc group (8.3% vs. 29.3%, p<0.001). This result was consistent in the multivariable analysis where patients' age and concomitant proton pump inhibitor use were adjusted. There was no case of gastric antral vascular ectasia (GAVE) in both groups. However, 29 (16.1%) patients in SSc group showed a clinically significant anemia (hemoglobin <10 mg/dL), with none of the endoscopic features showed significant associations with the outcome.
Go to : 
REFERENCES
2. Forbes A, Marie I. Gastrointestinal complications: the most frequent internal complications of systemic sclerosis. Rheumatology (Oxford). 2009; 48(Suppl 3):iii36–9.

3. Altman RD, Medsger TA Jr, Bloch DA, Michel BA. Predictors of survival in systemic sclerosis (scleroderma). Arthritis Rheum. 1991; 34:403–13.

4. Hunzelmann N, Genth E, Krieg T, Lehmacher W, Melchers I, Meurer M, et al. The registry of the German network for systemic scleroderma: frequency of disease subsets and patterns of organ involvement. Rheumatology (Oxford). 2008; 47:1185–92.

5. Alastal Y, Hammad TA, Renno A, Khalil B, Pierre J, Kwaah B, et al. Gastrointestinal manifestations associated with systemic sclerosis: results from the nationwide inpatient sample. Ann Gastroenterol. 2017; 30:498–503.

6. McFarlane IM, Bhamra MS, Kreps A, Iqbal S, Al-Ani F, Saladini-Aponte C, et al. Gastrointestinal manifestations of systemic sclerosis. Rheumatology (Sunnyvale). 2018; 8:pii: 235.

7. Savarino E, Furnari M, de Bortoli N, Martinucci I, Bodini G, Ghio M, et al. Gastrointestinal involvement in systemic sclerosis. Presse Med. 2014; 43:e279–91.

8. Watson M, Hally RJ, McCue PA, Varga J, Jiménez SA. Gastric antral vascular ectasia (watermelon stomach) in patients with systemic sclerosis. Arthritis Rheum. 1996; 39:341–6.

9. Ghrénassia E, Avouac J, Khanna D, Derk CT, Distler O, Suliman YA, et al. Prevalence, correlates and outcomes of gastric antral vascular ectasia in systemic sclerosis: a EUSTAR case-control study. J Rheumatol. 2014; 41:99–105.

10. Hung EW, Mayes MD, Sharif R, Assassi S, Machicao VI, Hosing C, et al. Gastric antral vascular ectasia and its clinical correlates in patients with early diffuse systemic sclerosis in the SCOT trial. J Rheumatol. 2013; 40:455–60.

11. Marie I, Levesque H, Ducrotté P, Denis P, Hellot MF, Benichou J, et al. Gastric involvement in systemic sclerosis: a prospective study. Am J Gastroenterol. 2001; 96:77–83.

12. van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2013; 72:1747–55.

13. LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988; 15:202–5.
14. Rey JF, Lambert R. ESGE Quality Assurance Committee. ESGE recommendations for quality control in gastrointestinal endoscopy: guidelines for image documentation in upper and lower GI endoscopy. Endoscopy. 2001; 33:901–3.

15. Aabakken L, Barkun AN, Cotton PB, Fedorov E, Fujino MA, Ivanova E, et al. Standardized endoscopic reporting. J Gastroenterol Hepatol. 2014; 29:234–40.

16. Marques S, Bispo M, Pimentel-Nunes P, Chagas C, Dinis-Ribeiro M. Image documentation in gastrointestinal endoscopy: review of recommendations. GE Port J Gastroenterol. 2017; 24:269–74.

17. Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med. 2002; 21:2409–19.

18. Kanfer EJ, Nicol BA. Haemoglobin concentration and erythrocyte sedimentation rate in primary care patients. J R Soc Med. 1997; 90:16–8.

19. Tiev KP, Cabane J. Digestive tract involvement in systemic sclerosis. Autoimmun Rev. 2011; 11:68–73.

20. Sung JJ, Kuipers EJ, El-Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther. 2009; 29:938–46.

21. Kim HS, Baik SJ, Kim KH, Oh CR, Lee JH, Jo WJ, et al. Prevalence of and risk factors for gastrointestinal diseases in Korean Americans and native Koreans undergoing screening endoscopy. Gut Liver. 2013; 7:539–45.

23. Ntoumazios SK, Voulgari PV, Potsis K, Koutis E, Tsifetaki N, Assimakopoulos DA. Esophageal involvement in scleroderma: gastroesophageal reflux, the common problem. Semin Arthritis Rheum. 2006; 36:173–81.

24. Denaxas K, Ladas SD, Karamanolis GP. Evaluation and management of esophageal manifestations in systemic sclerosis. Ann Gastroenterol. 2018; 31:165–70.

27. Ebert EC, Hagspiel KD. Gastrointestinal and hepatic manifestations of rheumatoid arthritis. Dig Dis Sci. 2011; 56:295–302.

28. Kuo CF, Luo SF, Yu KH, Chou IJ, Tseng WY, Chang HC, et al. Cancer risk among patients with systemic sclerosis: a nationwide population study in Taiwan. Scand J Rheumatol. 2012; 41:44–9.

29. Kang KY, Yim HW, Kim IJ, Yoon JU, Ju JH, Kim HY, et al. Incidence of cancer among patients with systemic sclerosis in Korea: results from a single centre. Scand J Rheumatol. 2009; 38:299–303.

30. Hashimoto A, Arinuma Y, Nagai T, Tanaka S, Matsui T, Tohma S, et al. Incidence and the risk factor of malignancy in Japanese patients with systemic sclerosis. Intern Med. 2012; 51:1683–8.

31. Joo YE, Park HK, Myung DS, Baik GH, Shin JE, Seo GS, et al. Prevalence and risk factors of atrophic gastritis and intestinal metaplasia: a nationwide multicenter prospective study in Korea. Gut Liver. 2013; 7:303–10.

32. Thonhofer R, Siegel C, Trummer M, Graninger W. Early endoscopy in systemic sclerosis without gastrointestinal symptoms. Rheumatol Int. 2012; 32:165–8.

33. Shibukawa G, Irisawa A, Sakamoto N, Takagi T, Wakatsuki T, Imamura H, et al. Gastric antral vascular ectasia (GAVE) associated with systemic sclerosis: relapse after endoscopic treatment by argon plasma coagulation. Intern Med. 2007; 46:279–83.

Go to : 
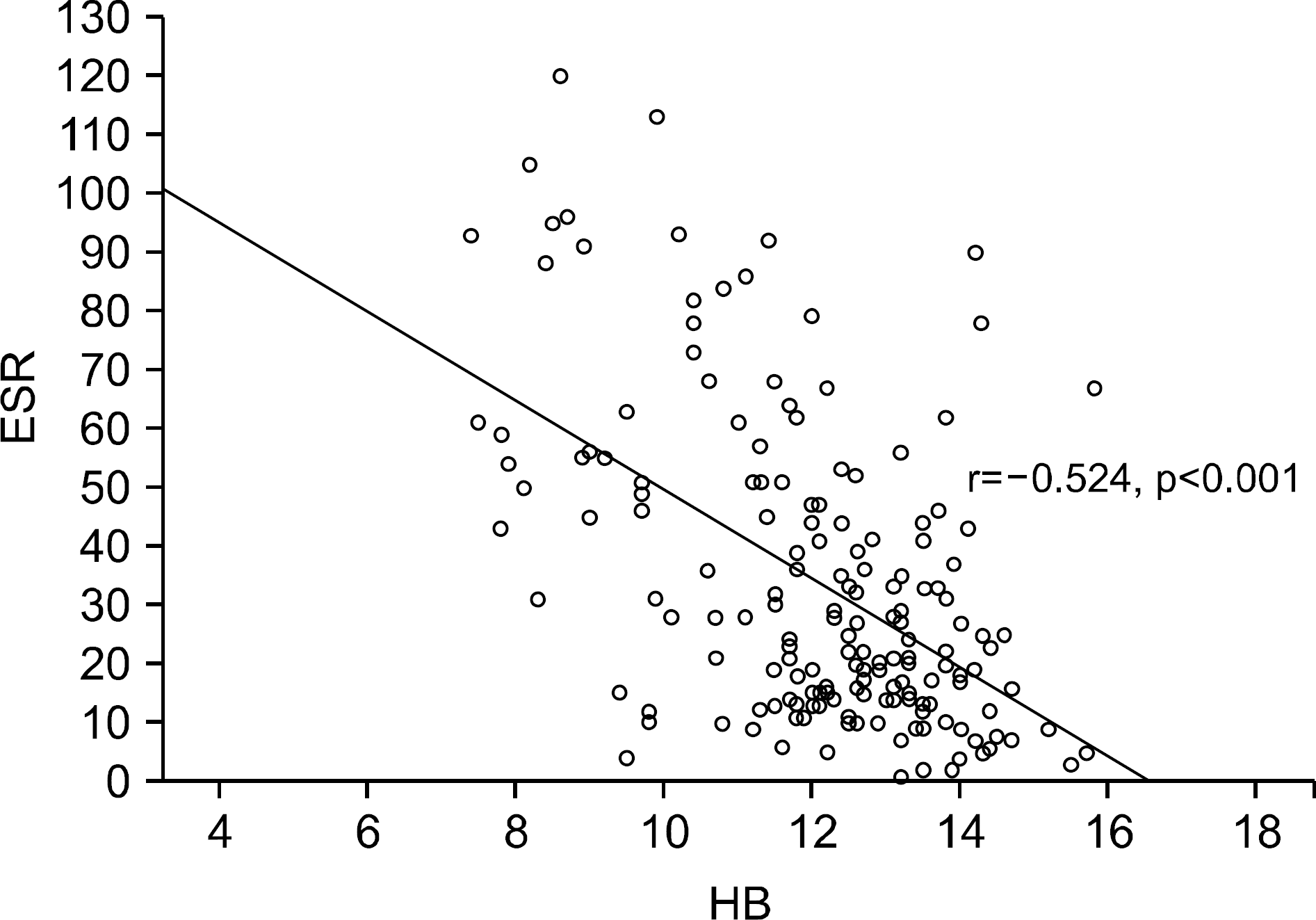 | Supplementary Figure 1.Scatter plot indicating the degree of relationship between the ESR and hemoglobin levels. ESR: erythrocyte sedimentation rate, HB: hemoglobin. |
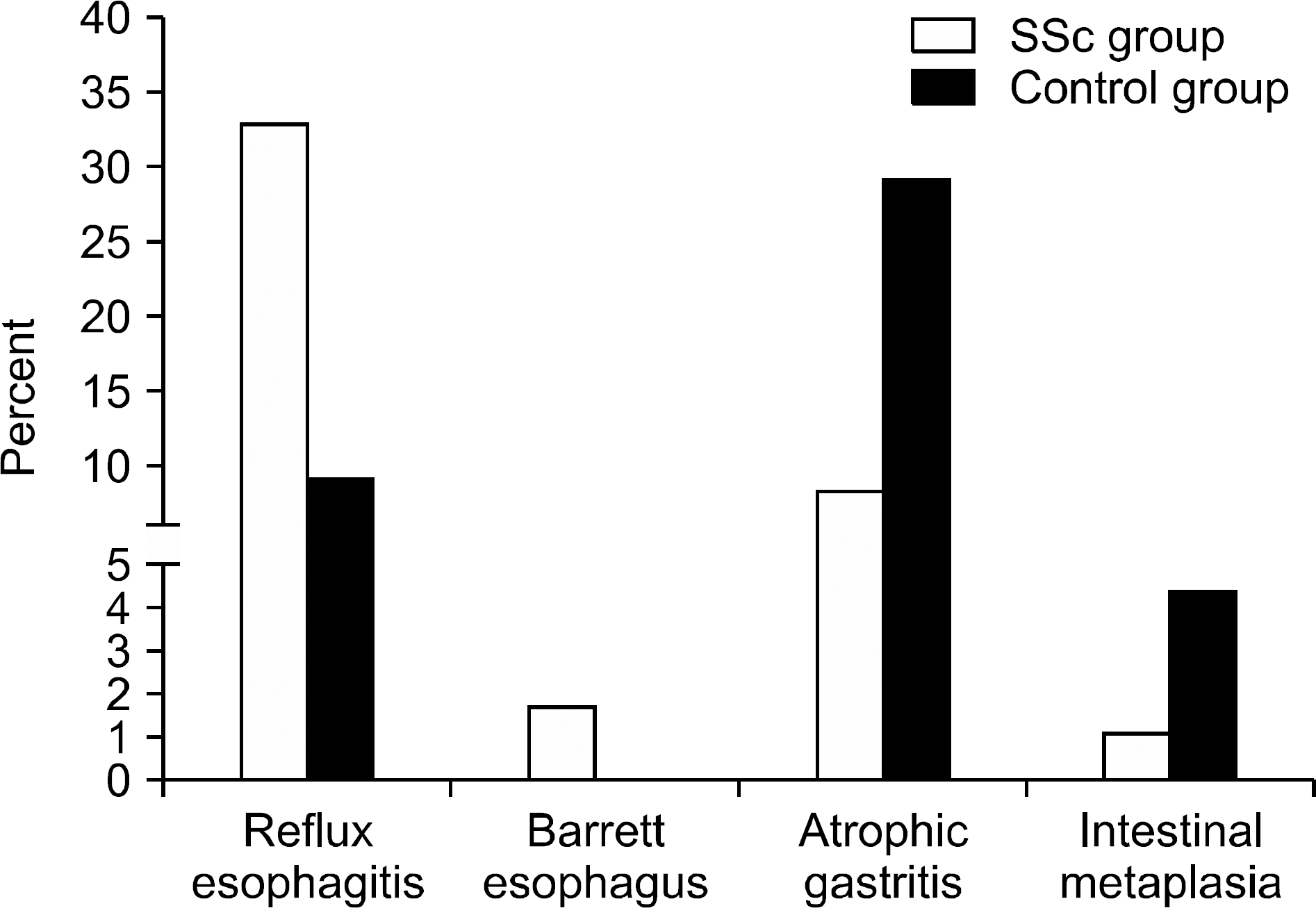 | Figure 1.Prevalence of reflux esophagitis, atrophic gastritis and their advanced lesions between the systemic sclerosis (SSc) and the control groups. |
Supplementary Table 1.
Reliability of endoscopic evaluation between the two readers
Supplementary Table 2.
Prevalence of endoscopic lesion between the two groups in the subgroup of the patients without PPI
Supplementary Table 3.
Prevalence of endoscopic lesion between the two groups after excluding the PACS data with low resolution which was taken before 2007
| Endoscopic lesions | Control group (n=181) | SSc group (n=158) | p-value |
|---|---|---|---|
| Atrophic gastritis | 53 (29.3) | 15 (9.3) | <0.001 |
| Intestinal metaplasia | 8 (4.4) | 2 (1.3) | 0.087 |
Table 1.
Clinical features of the included patients with systemic sclerosis (n=180)
| Clinical feature | Value |
|---|---|
| Age (yr) | 53.3±11.8 |
| Male sex | 23 (12.8) |
| Time from the onset of Raynaud phenomenon (yr) | 5.6±6.6 |
| Time from the onset of non-Raynaud phenomenon symptom (yr) | 3.4±4.0 |
| Disease duration (yr), (n=179) | 2.9±3.7 |
| Disease duration less than 5 years (n=179) 1 | 140 (77.8) |
| Ever-smokers | 15 (8.3) |
| Diffuse subtype | 88 (48.9) |
| Limited subtype | 92 (51.1) |
| Anti-scl70 antibody positive (n=171) | 72 (48.0) |
| Anti-centromere antibody positive (n=171) | 38 (25.3) |
| Anti-U1RNP antibody positive (n=154) | 23 (16.9) |
| Telangiectasia (n=145) | 32 (22.1) |
| Interstitial lung disease 1 | 110 (61.1) |
| GI manifestations | |
| Dysphasia | 44 (24.4) |
| Reflux | 72 (40.0) |
| Early satiety | 45 (25.0) |
| Vomiting | 10 (5.6) |
| Diarrhea | 29 (16.1) |
| Constipation | 13 (7.2) |
| Abdominal pain | 26 (14.4) |
| Melena | 1 (0.6) |
| Anemia* | 73 (40.6) |
| Significant anemia† | 29 (19.1) |
| NSAID use at baseline (n=179) | 27 (15.1) |
| Steroid use at baseline (n=179) | 59 (33.0) |
| PPI use at baseline (n=170) 1 | 101 (56.1) |
| ESR (mm/hr) | 34.0±26.1 |
Table 2.
Prevalence of endoscopic lesion between the two groups
Table 3.
Clinical features associated with atrophic gastritis on baseline EGD
| Variable | Univariable analysis | Multivariable analysis* | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value | |
| Age | 1.06 (1.03 to 1.09) | <0.001 | 1.06 (1.04 to 1.09) | <0.001 |
| Male sex | 1.05 (0.48 to 2.31) | 0.892 | † | |
| Disease duration | 0.95 (0.81 to 1.12) | 0.532 | † | |
| Ever-smokers | 0.31 (0.27 to 2.44) | 0.707 | † | |
| PPI use at baseline | 0.35 (0.17 to 0.72) | <0.001 | 1.23 (0.46 to 3.26) | 0.681 |
| Systemic sclerosis (vs. normal control) | 0.22 (0.12 to 0.410 | <0.001 | 0.17 (0.07 to 0.38) | <0.001 |
Table 4.
Clinical features associated with significant anemia at baseline




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download