Abstract
Objective
Methods
Results
REFERENCES
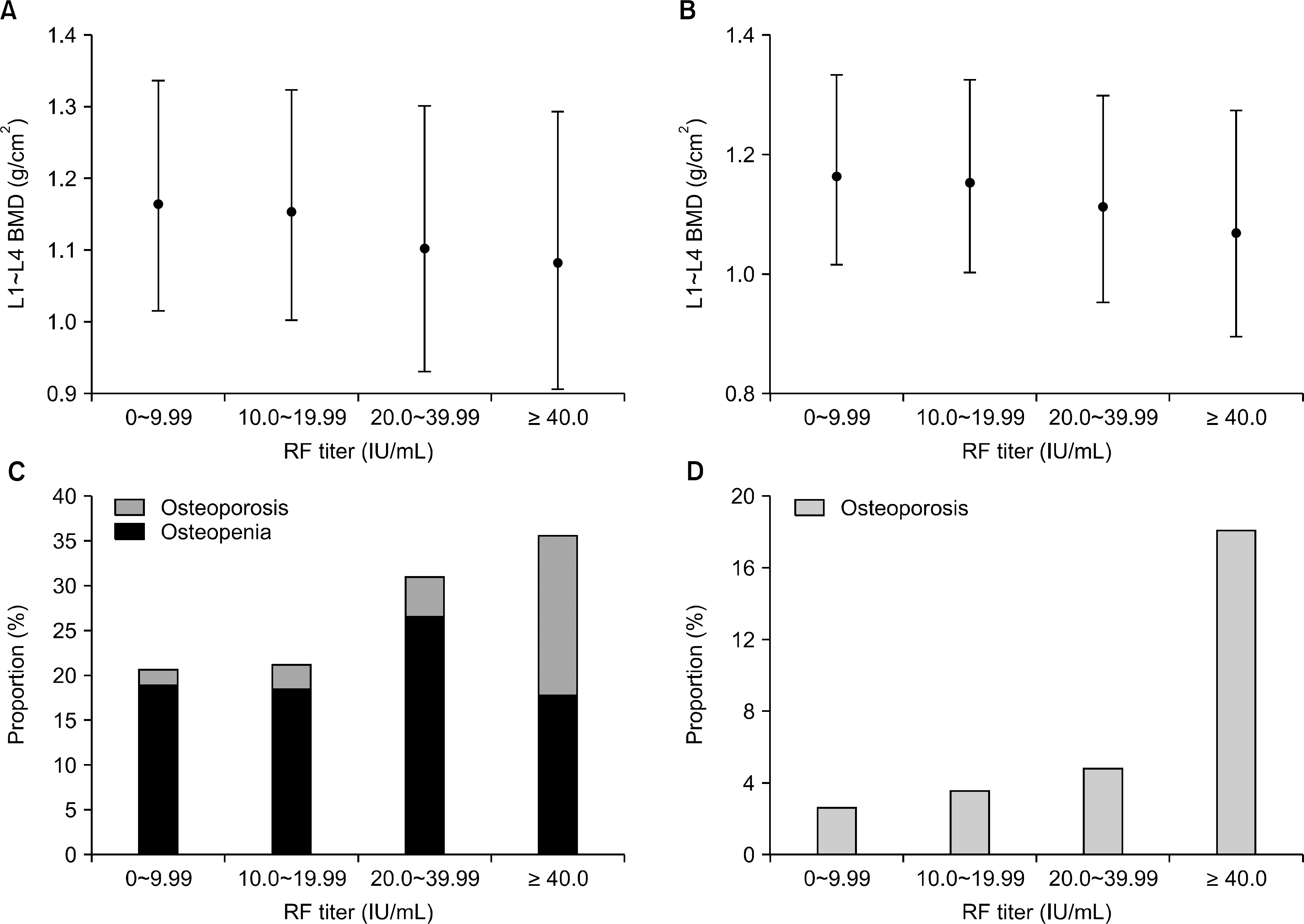 | Figure 2.Mean BMD and the proportion of low bone mass in lumbar spine along RF titers. (A) and (C) drawn for total subjects, and (B) and (D) for HBsAg-negative subjects. (A, B) RF titers were grouped into four categories, and each rhombus dot represents the mean estimated value (±95% CI) of lumbar BMD in the corresponding RF titer group. The imaginary connecting line between the dots demonstrates the decreasing tendency of BMD as the RF titers increase on one-way ANOVA testing (p=0.015 in A) and p=0.027 in B, respectively). (C, D) Each column represents the percentage of subjects with osteopenia (dark gray) and osteoporosis (light gray). Across the four groups, low bone mass frequency increases as RF titers increase (p for trend <0.001, both). BMD: bone mineral density, RF: rheumatoid factor, HBsAg: hepatitis B surface antigen, CI: confidence intervals. |
Table 1.
Values are presented as mean±standard deviation, number of subjects with percentages or median (interquartile range). RF: rheumatoid factor, HBsAg: hepatitis B virus surface antigen, HCV Ab: antibody against hepatitis C virus, eGFR: estimated glomerular filtration rate, CRP: C-reactive protein. p-values were determined by Chi-square test for categorical variables, and Student's t-test or Mann-Whitney U-test which was for skewed continuous variables.
Table 2.
| Variable | Lumbar spine | Variable | Total hip | ||||
|---|---|---|---|---|---|---|---|
| RF(−)(n=1,140) | RF(+)(n=54) | p-value | RF(−)(n=249) | RF(+)(n=12) | p-value | ||
| BMD (g/cm2) | 1.17±0.16 | 1.10±0.18 | 0.002 | BMD (g/cm2) | 0.97±0.15 | 0.94±0.23 | 0.537 |
| (n=1,194)* | (n=261)* | ||||||
| T-score (n=1,194)* | −0.05±1.33 | −0.64±1.52 | 0.009 | T-score (n=261)* | 0.17±1.19 | −0.07±1.76 | 0.116 |
| Category based on | <0.001 | Category based on | 0.683 | ||||
| spine T-score | total hip T-score | ||||||
| (n=1,386)* | (n=307)* | ||||||
| Normal | 1,043 (78.9) | 47 (73.4) | Normal | 246 (83.4) | 9 (75.0) | ||
| Osteopenia | 241 (18.2) | 9 (14.1) | Osteopenia | 47 (15.9) | 3 (25.0) | ||
| Osteoporosis | 38 (2.9) | 8 (12.5) | Osteoporosis | 2 (0.7) | 0 | ||
Values are presented as mean±standard deviation or number of subjects with percentages. RF: rheumatoid factor, HBsAg: hepatitis B surface antigen, BMD: bone mineral density.
* The discrepancy of the numbers was due to the missing values between the L1∼ L4 BMD results and the category, and the category was determined by the lowest T-score. p-values were determined by Chi-square test for categorical variables, and Student's t-test or Mann-Whitney U-test for skewed continuous variables.
Table 3.
| Variable | Lumbar spine | Variable | Total hip | ||||
|---|---|---|---|---|---|---|---|
| RF(−)(n=1,090) | RF(+)(n=54) | p-value | RF(−)(n=240) | RF(+)(n=10) | p-value | ||
| BMD (g/cm2) | 1.17±0.16 | 1.10±0.18 | 0.004 | BMD (g/cm2) | 0.97±0.15 | 0.96±0.24 | 0.922 |
| (n=1,135)† | (n=250)† | ||||||
| T-score (n=1,135)† | −0.06±1.32 | −0.64±1.47 | 0.004 | T-score (n=250)† | 0.17±1.19 | −0.15±1.85 | 0.951 |
| Category based on | 0.026 | Category based on | 0.769 | ||||
| spine T-score | total hip T-score | ||||||
| (n=1,319)† | (n=262)† | ||||||
| Normal | 1,002 (79.2) | 39 (72.2) | Normal | 215 (85.0) | 7 (77.8) | ||
| Osteopenia | 227 (17.9) | 9 (16.7) | Osteopenia | 36 (14.2) | 2 (22.2) | ||
| Osteoporosis | 36 (2.8) | 6 (11.1) | Osteoporosis | 2 (0.8) | 0 | ||
Values are presented asmean±standard deviation or number of subjects with percentages. RF: rheumatoid factor, HBsAg: hepatitis B surface antigen, BMD: bone mineral density. p-values were determined by Chi-square test for categorical variables, and Student's t-test or Mann-Whitney U-test for skewed continuous variables.
Table 4.
| BMD (g/cm2) | B | SE | p-value | R2 |
|---|---|---|---|---|
| Model 1 | −0.065 | 0.023 | 0.005 | 0.101 |
| Model 2 | −0.069 | 0.028 | 0.014 | 0.084 |
| Model 3 | −0.088 | 0.035 | 0.011 | 0.147 |
Model 1: adjusted for age, body mass index. Model 2: as for Model 1 plus: alcohol intake (g/week), smoking (pack-years), history of hypertension, and history of coronary artery disease. Model 3: as for Model 2 plus: fasting glucose, alanine aminotransferase, estimated glomerular filtration rate, uric acid, triglyceride, high density lipoprotein, homocysteine, ferritin, total vitamin D, and serum concentration of calcium and phosphorus. RF: rheumatoid factor, BMD: bone mineral density, B: unstandardized coefficient means the degree of change in lumbar spine BMD values (g/cm2) along the presence of RF, SE: standard error, R2: coefficient of multiple determination.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download