Abstract
Objective
Evaluate effectiveness/safety of tacrolimus in patients in Korea with active rheumatoid arthritis (RA) and unsuccessful response to disease-modifying anti-rheumatic drugs (DMARDs).
Methods
Open-label, single-arm, non-comparative, 24-week, Phase-IV study in patients with active RA who had taken DMARDs for >6 months. Following a washout period, tacrolimus was initiated (baseline–12 weeks; dose 2 mg/day and 1.5 mg/day in patients aged ≤65 and >65 years, respectively). After 12 weeks, dose could be adjusted (remaining between 1∼3 mg); treatment continued to 24 weeks. Primary endpoint was American College of Rheumatology 20% improvement (ACR20) (baseline– Week 24). Secondary endpoints included ACR50/ACR70 response, disease-activity score in 28 joints (DAS28) erythrocyte sedimentation rate (ESR), number of tender/swollen joints, and bone mineral density (BMD) loss. Adverse events (AEs) were recorded.
Results
Overall, 121 patients were analysed. Mean ±standard deviation tacrolimus dose baseline– Week 24 was 1.81±0.47 mg/day. After 24 weeks, 64.5%, 39.7%, and 19.0% of patients were ACR20, ACR50, and ACR70 responders, respectively. DAS28-ESR score decreased from 5.5±0.8 (baseline) to 3.7±1.5 (Week 24; p<0.0001); number of tender/swollen joints decreased. Between screening and Week 24, change in BMD-T score in lumbar and femur regions was −0.06±0.38 (p=0.1550) and −0.04±0.28 (p=0.0936), respectively, with no significant change in International Society for Clinical Densitometry classification. Fifty-six (46.3%) patients experienced 93 AEs; 75.3% were mild. No unexpected safety signals identified.
Go to : 
REFERENCES
2. Pincus T, Gibson KA, Block JA. Premature mortality: a neglected outcome in rheumatic diseases? Arthritis Care Res (Hoboken). 2015; 67:1043–6.

3. Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017; 76:960–77.
4. Katchamart W, Trudeau J, Phumethum V, Bombardier C. Efficacy and toxicity of methotrexate (MTX) monotherapy versus MTX combination therapy with non-biological disease-modifying antirheumatic drugs in rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2009; 68:1105–12.

5. Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. 2009; 68:1100–4.

6. Kvalvik AG, Lefsaker L, Dyvik S, Brun JG. Anti-tumor necrosis factor-alpha therapy in the ordinary clinical setting: three-year effectiveness in patients with rheumatoid arthritis. Joint Bone Spine. 2007; 74:606–11.

7. Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003; 48:35–45.
8. Gizinski AM, Fox DA. T cell subsets and their role in the pathogenesis of rheumatic disease. Curr Opin Rheumatol. 2014; 26:204–10.

9. Scott LJ, McKeage K, Keam SJ, Plosker GL. Tacrolimus: a further update of its use in the management of organ transplantation. Drugs. 2003; 63:1247–97.
10. Ogawa T, Tokuda M, Tomizawa K, Matsui H, Itano T, Konishi R, et al. Osteoblastic differentiation is enhanced by rapamycin in rat osteoblast-like osteosarcoma (ROS 17/2.8) cells. Biochem Biophys Res Commun. 1998; 249:226–30.

11. Spolidorio LC, Nassar PO, Nassar CA, Spolidorio DM, Muscará MN. Conversion of immunosuppressive monotherapy from cyclosporin A to tacrolimus reverses bone loss in rats. Calcif Tissue Int. 2007; 81:114–23.

12. Lee WS, Lee SI, Lee MS, Kim SI, Lee SS, Yoo WH. Efficacy and safety of low-dose tacrolimus for active rheumatoid arthritis with an inadequate response to methotrexate. Korean J Intern Med. 2016; 31:779–87.

13. Kawai S, Takeuchi T, Yamamoto K, Tanaka Y, Miyasaka N. Efficacy and safety of additional use of tacrolimus in patients with early rheumatoid arthritis with inadequate response to DMARDs − a multicenter, double-blind, paral-lel-group trial. Mod Rheumatol. 2011; 21:458–68.
14. Kawai S, Yamamoto K. Safety of tacrolimus, an immunosuppressive agent, in the treatment of rheumatoid arthritis in elderly patients. Rheumatology (Oxford). 2006; 45:441–4.

15. Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992; 35:498–502.

16. van der Heijde DM, van't Hof MA, van Riel PL, Theunisse LA, Lubberts EW, van Leeuwen MA, et al. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis. 1990; 49:916–20.

17. Kawai S, Hashimoto H, Kondo H, Murayama T, Kiuchi T, Abe T. Comparison of tacrolimus and mizoribine in a randomized, double-blind controlled study in patients with rheumatoid arthritis. J Rheumatol. 2006; 33:2153–61.
18. Furst DE, Saag K, Fleischmann MR, Sherrer Y, Block JA, Schnitzer T, et al. Efficacy of tacrolimus in rheumatoid arthritis patients who have been treated unsuccessfully with methotrexate: a six-month, double-blind, randomized, doseranging study. Arthritis Rheum. 2002; 46:2020–8.

19. Kondo H, Abe T, Hashimoto H, Uchida S, Irimajiri S, Hara M, et al. Efficacy and safety of tacrolimus (FK506) in treatment of rheumatoid arthritis: a randomized, double blind, placebo controlled dose-finding study. J Rheumatol. 2004; 31:243–51.
20. Kremer JM, Habros JS, Kolba KS, Kaine JL, Borton MA, Mengle-Gaw LJ, et al. Tacrolimus in rheumatoid arthritis patients receiving concomitant methotrexate: a six-month, open-label study. Arthritis Rheum. 2003; 48:2763–8.
21. Yocum DE, Furst DE, Kaine JL, Baldassare AR, Stevenson JT, Borton MA, et al. Efficacy and safety of tacrolimus in patients with rheumatoid arthritis: a double-blind trial. Arthritis Rheum. 2003; 48:3328–37.

22. Takeuchi T, Ishida K, Shiraki K, Yoshiyasu T. Safety and effectiveness of tacrolimus add-on therapy for rheumatoid arthritis patients without an adequate response to biological disease-modifying anti-rheumatic drugs (DMARDs): postmarketing surveillance in Japan. Mod Rheumatol. 2018; 28:48–57.

23. Miyazaki M, Fujikawa Y, Takita C, Tsumura H. Tacrolimus and cyclosporine A inhibit human osteoclast formation via targeting the calcineurindependent NFAT pathway and an activation pathway for c-Jun or MITF in rheumatoid arthritis. Clin Rheumatol. 2007; 26:231–9.

24. Bae SC, Cook EF, Kim SY. Psychometric evaluation of a Korean Health Assessment Questionnaire for clinical research. J Rheumatol. 1998; 25:1975–9.
Go to : 
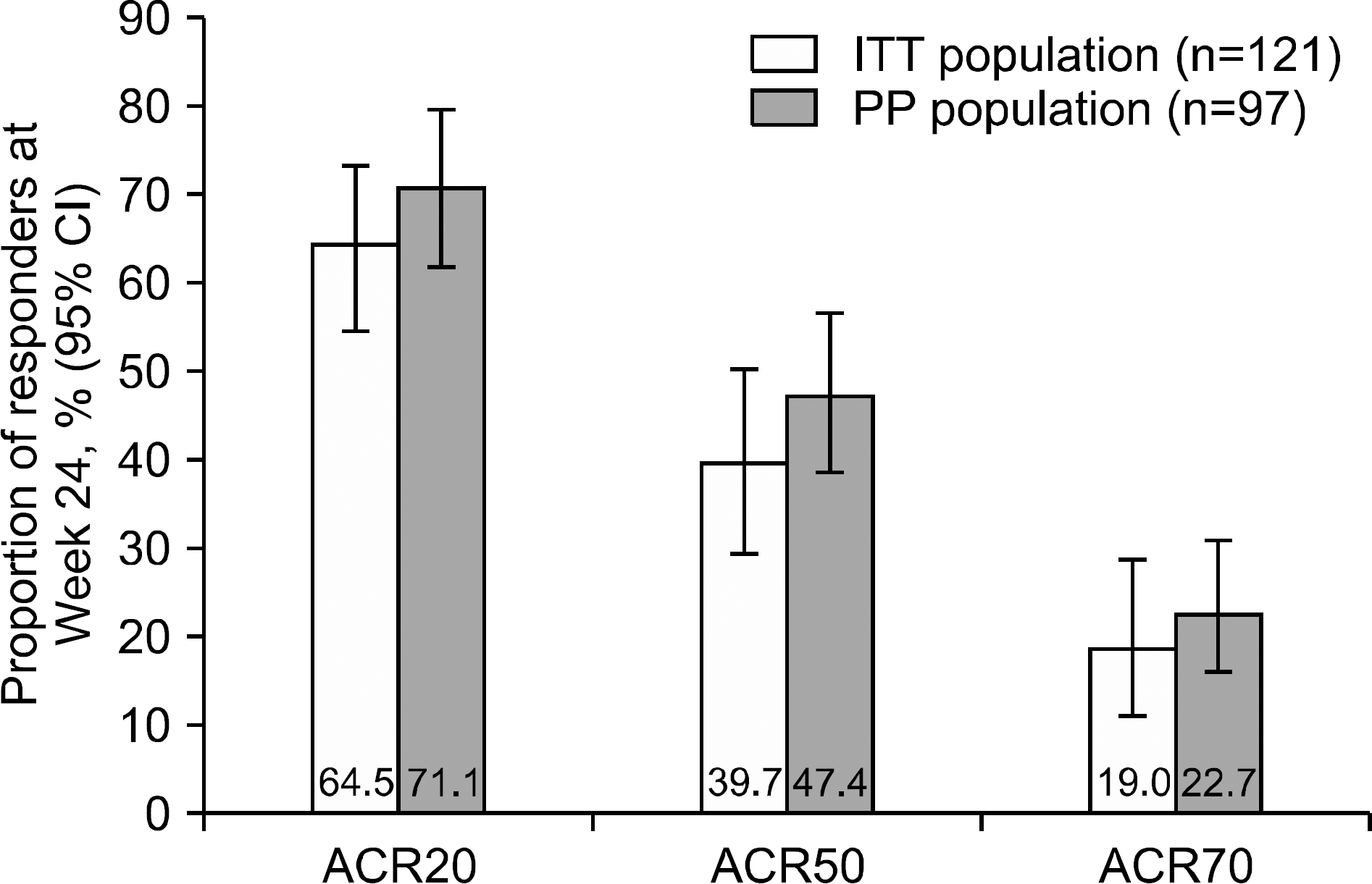 | Figure 2.Proportion of ACR responders at Week 24 in the ITT and PP populations after 24 weeks of treatment with tacrolimus. ACR20, ≥20% improvement in the seven ACR response criteria. ACR50, ≥50% improvement in the seven ACR response criteria. ACR70, ≥70% improvement in the 7 ACR response criteria. ACR: American College of Rheumatology, ITT: intention-to-treat, PP: per-protocol, CI: confidence interval. |
Table 1.
Patient demographics and baseline characteristics, overall and stratified by patient age (≤65 years vs. >65 years) (ITT population)
| Characteristics | Patients aged ≤65 years (n=106) | Patients aged >65 years (n=15) | All patients (n=121) |
|---|---|---|---|
| Female sex | 93 (87.7) | 14 (93.3) | 107 (88.4) |
| Age (yr) | 52.7±8.5 | 69.3±2.6 | 54.7±9.7 |
| Height (cm) | 157.3±6.6 | 152.7±6.4 | 156.7±6.7 |
| Weight (kg) | 57.4±9.4 | 54.3±7.9 | 57.0±9.3 |
| BMI (kg/m2) | 23.2±3.6 | 23.3±3.0 | 23.2±3.5 |
| Duration of RA (mo) ACR functional class | 87.0±73.8 | 136.1±108.5 | 93.1±80.0 |
| Class I | 4 (3.8) | 0 (0) | 4 (3.3) |
| Class II | 94 (88.7) | 10 (66.7) | 104 (86.0) |
| Class III | 6 (5.7) | 5 (33.3) | 11 (9.1) |
| Class IV | 2 (1.9) | 0 (0.0) | 2 (1.7) |
| DAS28-ESR score | – | – | 5.5±0.8 |
| BMD-T score* | |||
| Lumbar region | – | – | −1.43±1.34 |
| Femur region | – | – | −1.20±1.11† |
| ESR (mm/h) | – | – | 42.4±22.5 |
| CRP (mg/dL) | – | – | 1.30±1.28 |
| Bone turnover markers | |||
| Bone-specific alkaline phosphatase Normal | – | – | 81 (68.1) |
| Abnormal (non-significant) | – | – | 35 (29.4) |
| Abnormal (clinically significant) | – | – | 3 (2.5) |
| N (missing) | – | – | 119 (2) |
| Osteocalcin | |||
| Normal | – | – | 94 (79.0) |
| Abnormal (non-significant) | – | – | 25 (21.0) |
| Abnormal (clinically significant) | – | – | 0 (0.0) |
| N (missing) | – | – | 119 (2) |
| C-telopeptide | |||
| Normal | – | – | 117 (98.3) |
| Abnormal (non-significant) | – | – | 2 (1.7) |
| Abnormal (clinically significant) | – | – | 0 (0.0) |
| N (missing) | – | – | 119 (2) |
| Receptor activator of NF-B | |||
| Normal | – | – | 4 (100.0) |
| Abnormal (non-significant) | – | – | 0 (0.0) |
| Abnormal (clinically significant) | – | – | 0 (0.0) |
| N (missing) | – | – | 4 (117) |
| Osteoprotegerin | |||
| Normal | – | – | 4 (100.0) |
| Abnormal (non-significant) | – | – | 0 (0.0) |
| Abnormal (clinically significant) | – | – | 0 (0.0) |
| N (missing) | – | – | 4 (117) |
| Prior DMARDs/biological agents DMARDs | 106 (100.0) | 15 (100.0) | 121 (100.0) |
| Methotrexate | 97 (91.5) | 14 (93.3) | 111 (91.7) |
| Hydroxychloroquine | 46 (43.3) | 5 (33.3) | 51 (42.1) |
| Sulfasalazine | 41 (38.7) | 5 (33.3) | 46 (38.0) |
| Leflunomide | 6 (5.7) | 1 (6.7) | 7 (5.8) |
| Others | 12 (11.3) | 3 (20.0) | 15 (12.4) |
| Biological agents | 3 (2.8) | 1 (6.7) | 4 (3.3) |
| Infliximab | 2 (1.9) | 0 (0.0) | 2 (1.7) |
| Etanercept | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Adalimub | 1 (0.9) | 1 (6.7) | 2 (1.7) |
| Rituximab | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| RA medications other than DMARDs/biological agents | 101 (95.3) | 14 (93.3) | 115 (95.0) |
Values are presented as number (%) or mean±standard deviation unless otherwise stated. ITT: intention-to-treat, BMI: body mass index, RA: rheumatoid arthritis, ACR: American College of Rheumatology, DAS28: disease activity score in 28 joints, ESR: erythrocyte sedimentation rate, BMD: bone mineral density, CRP: C-reactive protein, NF- B: nuclear factor B, DMARD: disease-modifying anti-rheumatic drugs.
Table 2.
DAS28-ESR response by EULAR classifications at Week 24, in the ITT and PP populations
Table 3.
Summary of BMD-T score at screening versus Week 24, and BMD-T score classification by ISCD guideline, for the ITT and PP populations
| BMD-T score | ITT population | PP population |
|---|---|---|
| Lumbar vertebra region (n) | 121 | 97 |
| Screening | −1.43±1.34 | −1.35±1.31 |
| Week 24 | −1.50±1.35 | −1.42±1.34 |
| Difference from screening to Week 24 | −0.06±0.38 | −0.06±0.33 |
| p-value* | 0.1550 | 0.1138 |
| Transitioned from normal/osteopenia to osteoporosis (screening– Week 24) | 2 (1.7) | 1 (1.0) |
| Transitioned from osteoporosis to normal/osteopenia (screening– Week 24) | 4 (3.3) | 3 (3.1) |
| p-value† | 0.6875 | 0.6250 |
| Femur region (n) | 120 | 96 |
| Screening | −1.20±1.11 | −1.10±1.10 |
| Week 24 | −1.24±1.08 | −1.16±1.08 |
| Difference from baseline to Week 24 | −0.04±0.28 | −0.06±0.26 |
| p-value* | 0.0936 | 0.0483 |
| Transitioned from normal/osteopenia to osteoporosis (screening– Week 24) | 2 (1.7) | 1 (1.0) |
| Transitioned from osteoporosis to normal/osteopenia (screening– Week 24) | 2 (1.7) | 1 (1.0) |
| p-value† | 1.000 | 1.000 |
Table 4.
Incidence of AEs experienced by ≥1% of patients in the SAF, by system organ class and preferred term




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download