Abstract
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease affecting various organs. Among its manifestations, inflammatory pseudotumor (IPT) is an extremely rare disease about which no case has been reported of it occurring in the liver. We present a case of a SLE patient with hepatic IPT (hIPT) successfully treated with immunosuppressants. A 16-year-old male with elevated liver enzymes visited our clinic and was diagnosed as SLE. Although no lesion was observed in the initial abdomen ultrasonography, the abdominal CT on hospital day 7 revealed a new hepatic mass resembling an abscess. Despite 5 weeks of antibiotics treatment, the hepatic mass remained, and was re-diagnosed as hIPT secondary to SLE with an abdominal MRI. After high dose prednisolone and mycophenolate mofetil treatment, lupus activity subsided and hIPT disappeared in the follow-up CT. This case suggests that hIPT should be considered as a differential diagnosis among hepatic mass in SLE patients.
Inflammatory pseudotumor (IPT) is a rare benign neoplasm often described as a discrete, localized mass composed of a benign proliferation of inflammatory cells with fibrosis, which mimics a malignant neoplasm. Since the term “inflammatory pseudotumor” appeared in 1954, IPT has been reported in various organs, including the liver, lymph nodes, and soft tissues [1]. Its etiology and pathogenesis remain unknown.
Infection is frequently considered as main cause of IPT as antibiotic treatment cases have been reported to have contributed to the remission of IPTs [2]. IPTs were also suspected to be associated with autoimmune disorders like primary sclerosing cholangitis, rheumatoid arthritis, and Crohn's disease [34].
Systemic lupus erythematosus (SLE) encompasses a broad spectrum of liver diseases, from asymptomatic serum transaminase elevation to other autoimmune diseases such as autoimmune hepatitis or primary biliary cirrhosis. Viral hepatitis and drug-induced hepatotoxicity also cause comorbidity in SLE [5]. Reported cases of IPTs are extremely rare in SLE [67]. This paper describes an unusual case of hepatic IPT (hIPT) that occurred in a patient with active SLE.
A 16-year old male with an oral ulcer, malar rash, and polyarthralgia was referred to our hospital. At the initial evaluation, he had bicytopenia (hemoglobin 10.9 g/dL, white blood cell [WBC] 2.4×109/L, eosinophil 0.012×109/L), elevated liver enzymes (aspartate aminotransferase 132 U/L, alanine aminotransferase 155 U/L), and proteinuria (24 hours urine protein 816.4 mg/day). Low complement level (C3 28 mg/dL, C4 2 mg/dL), anti-nuclear antibody titer (>1:2,560), anti-double-stranded DNA auto-antibody (anti-dsDNA) titer (>1:1,280), and positive anti-Smith antibody were sufficient to meet 2012 Systemic Lupus International Collaborating Clinics criteria to warrant a diagnosis of SLE with SLE Disease Activity Index score 18. Anticardiolipin antibody, lupus anticoagulant, and antineutrophil cytoplasmic antibodies (ANCA) were all negative. Initial abdomen ultrasonography revealed highly echogenic liver parenchyma and renal medullae without abnormal lesion.
On hospital day 2, an abrupt fever (38.2℃) developed, and laboratory examinations were performed. Serum inflammatory markers in the following 5 days (C-reactive protein [CRP] 69.0 mg/L, erythrocyte sedimentation rate [ESR] 122 mm/h) showed significant elevation. However, blood gram stain, viral hepatitis lab, and cerebrospinal fluid analysis results were negative, and liver enzymes returned to normal range. To assess inflammatory focus, abdominopelvic computed tomography (APCT) was performed on hospital day 7; disclosing multiple low-density lesions on the right hepatic lobe with peripheral enhancement in the arterial phase (Figure 1). There was no abnormal finding in other organs. Based on computed tomography (CT) findings, abscess or metastatic cancer was highly suspected. However, metastatic cancer was unlikely as extrahepatic lesion was absent and the initial abdomen ultrasonography result revealed no mass like lesions. We initiated metronidazole and ceftriaxone administration, and decreased the daily dose of prednisolone from 10 mg to 5 mg. Intravenous antibiotic treatment lasted for a total of 16 days; a follow-up CT was taken on hospital day 18, revealing unchanged hepatic mass (Figure 1). Despite negative blood culture results and lowering peak body temperature (<38℃), CT findings and high ESR aroused suspicion of a hepatic pseudotumor secondary to lupus activity. On hospital day 21, we decided to increase the prednisolone dose to 20 mg/day, switch to oral antibiotics, and discharge the patient.
One week after being discharged, he was examined in the outpatient department. He had no fever during his discharge period, and CRP returned to normal range. However, ESR (107 mm/h) and proteinuria (urine protein/creatinine ratio [PCR] 1,043.1 mg/g) had increased. After continuing on antibiotics and prednisolone of 20 mg/day for another two weeks, we observed that his ESR was still high (88 mm/h) and proteinuria had aggravated (urine PCR 1,653.8 mg/g, protein 3+, WBC 20~29/high power field [HPF] in urinalysis). For further work up, liver magnetic resonance imaging (MRI) was taken.
From the liver MRI, the size of the largest mass was still more than half the size of the original CT image. Lesions were slightly hypointense on T1-weighted and hyperintense on T2-weighted images. Infiltrating inflammatory tissues were seen, and intact portal veins were visible inside. The lesion had subtle high signal intensity on the axial diffusion weighted image (DWI) on high b-values (b=800 s/mm2) and low signal intensity on the apparent diffusion coefficient (ADC) map, indicating mild diffuse restriction. In dynamic contrast-enhanced MRI, the lesion was isointense on portal-venous phase and showed gradual enhancement of internal septation from equilibrium to hepatobiliary phase (Figure 2). Such a radiologic pattern did not match the usual findings of a mature liver abscess, which usually presents as a high DWI signal within the necrotic abscess cavity and high ADC values at the abscess rim. Overall MRI findings suggested nonspecific inflammatory lesion mimicking hepatic mass, rather than liver abscess. As the hepatic lesion was improving after steroid administration, we proceeded with a renal biopsy instead to evaluate the worsening proteinuria. The biopsy result was consistent with type IV lupus nephritis. Since immunosuppressive treatment was required to control active lupus nephritis, the prednisolone dosage was increased from 20 mg to 30 mg (0.5 mg/kg)/day, and mycophenolate mofetil (MMF) 1 g/day was started, while antibiotics were stopped.
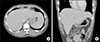
After changing the treatment, he remained in good condition. Follow-up APCT which was performed after 8 weeks of MMF treatment revealed complete disappearance of hepatic lesions (Figure 3). At 24 weeks after the first visit to our hospital, he was in a stable condition under a low dose (7.5 mg/day) of prednisolone and 2 g/day of MMF (Figure 4).
SLE includes various types of liver diseases. Abnormal liver function tests are seen in 23%~55% cases of SLE patients [8]. The liver-related complications in SLE include autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, viral hepatitis, drug induced hepatitis, and lymphomatous involvement of the liver [9].
Hepatic IPT, first described in 1953, usually presents with nonspecific symptoms such as fever, weight loss and general fatigue [3]. In general, hIPT is depicted as a localized mass composed of a benign proliferation of inflammatory cells with varying amounts of fibrosis or necrosis, which mimics features of an abscess or malignant neoplasm [1]. According to a multicenter study reporting 45 APCT scans of hIPT patients, 82.5% of hIPT were presented as poorly defined peripheral enhancement at the arterial phase and 77.0% as poorly defined hyperattenuating lesions with internal hypo-attenuating areas at the equilibrium phase, which highly resemble typical radiological findings of liver abscess [10].
Our case reports idiopathic hepatic mass found in an adolescent male who was diagnosed as SLE. Hepatic mass formations associated with SLE activity are rare [11]. Therefore, liver biopsies were considered essential to identify its characteristics. In our case, multiple hypodense hepatic lesions with rim enhancement on APCT, together with fever, pointed to the diagnosis of multiple liver abscesses. However, underlying SLE, negative culture results, non-response to antibiotics, high ESR, and steadily deteriorating proteinuria indicated the possibility of autoimmune related hepatic mass. The liver MRI findings helped to determine the characteristics of the hepatic mass. MRI pattens of our case differed from that of an abscess, where cellular tissues demonstrate strong intensity on higher b-value images due to restricted diffusion. Lack of necrotic core on DWI and absence of double target sign were also helpful in excluding the likelihood of liver abscess. Double target sign, the characteristic imaging feature of hepatic abscess peripheral rim, is denoted on T2-weighted images by an iso to hypointense inner layer and a hyperintense outer layer. Such MRI findings were not present in our case. Intact vasculature inside the mass even lowered possibility of liver abscess, as venous thrombophlebitis has been reported to accompany adjacent liver abscess as frequently as 42% in cases [12].
The possibility of vasculitis was also considered in this case. Vasculitis involving solely the liver in SLE has only been reported in a few published cases. Wangkaew et al. [11] reported a patient with SLE who had hepatic vasculitis with necrosis mimicking multiple liver abscesses as an initial presentation. However, it differed from our case as the lesions revealed no appreciable enhancement. Thietart et al. [13] reported a sequence of stenosis and aneurysmal dilatations of the hepatic arteries in their case report, but none of those findings corresponded to our case. Moreover, negative results of the vasculitis-related laboratory test reduced possibility of anti-phospholipid syndrome or ANCA-associated vasculitis.
Lastly, malignant tumors should be excluded. The CT findings of our case are also consistent with mass-forming Intrahepatic Cholangiocarcinoma (ICC). However, typical MRI findings of ICC such as lobulated shape, rim enhancement during the arterial phase, a target appearance with a peripheral hyperintense rim on DWI, and peripheral biliary dilatation were not found [14].
After excluding differential diseases, we diagnosed the lesion as hIPT, an inflammatory cell aggregate caused by an autoimmune entity in liver. On the MRI, a hIPT shows iso to lower signal intensity on T1-weighted images and slightly higher signal intensity on T2-weighted images. Its dynamic contrast-enhanced MRI is characterized by enhancement of the fibrous marginal zone or gradual internal septal enhancement toward the equilibrium phase [15]. In this patient, the lesion was accompanied by high lupus activity, which was totally resolved after 2 months of high dose prednisolone and MMF treatment.
To our knowledge, this is the first reported case of hIPT in SLE treated successfully with corticosteroid and MMF therapy without pathologic confirmation. In general, active liver biopsy was recommended for hIPT because it is difficult to distinguish from other diseases. However, thanks to advanced radiologic techniques, we could confidently suggest hIPT in this case without the need for invasive procedure. Based on this, we suggest that hIPT should be included as a major disease to be considered in SLE patients with idiopathic hepatic lesion mimicking an abscess.
Figures and Tables
Figure 1
(A) Initial contrast enhanced abdominopelvic computed tomography (CT) on hospital day (HD) 7 with transverse (left) and coronal (right) view showing rim-enhancing hypo-attenuated lesion at subcapsular area, largest lesion (arrows) measured 3.4×2.5 cm on segment 8. (B) Follow-up CT on HD 18 with transverse (left) and coronal (right) view revealing unchanged hepatic lesion.
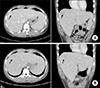
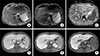
Figure 2
Follow-up transverse view of liver magnetic resonance imaging images on 5 weeks after antibiotics treatment, with (A) Axial T2-weighted fast spin echo, (B) diffusion weighted image on b=800 s/mm2, (C) apparent diffusion coefficient map, (D~F) portal-venous, equilibrium, and hepatobiliary phase. The largest lesion measured 2.6×2.0 cm on segment 8.
References
1. Patnana M, Sevrukov AB, Elsayes KM, Viswanathan C, Lubner M, Menias CO. Inflammatory pseudotumor: the great mimicker. AJR Am J Roentgenol. 2012; 198:W217–W227.

2. Ntinas A, Kardassis D, Miliaras D, Tsinoglou K, Dimitriades A, Vrochides D. Inflammatory pseudotumor of the liver: a case report and review of the literature. J Med Case Rep. 2011; 5:196.



3. Díaz-torné C, Narváez J, De Lama E, Diez-García M, Narváez JA, Bernad B, et al. Inflammatory pseudotumor of the liver associated with rheumatoid arthritis. Arthritis Rheum. 2007; 57:1102–1106.


4. Younis N, Khaleeli AA, Soran H, Monteith PG. Inflammatory pseudotumour of the liver associated with diabetes mellitus. Int J Clin Pract. 2001; 55:717–719.

5. Bessone F, Poles N, Roma MG. Challenge of liver disease in systemic lupus erythematosus: clues for diagnosis and hints for pathogenesis. World J Hepatol. 2014; 6:394–409.



6. Khatri A, Agrawal A, Sikachi RR, Mehta D, Sahni S, Meena N. Inflammatory myofibroblastic tumor of the lung. Adv Respir Med. 2018; 86:27–35.


7. Oh JS, Kwon GY, So MW, Choi SH, Kim YG, Nah SS, et al. A case of plasma cell granuloma of skull in a patient with systemic lupus erythematosus. J Korean Rheum Assoc. 2006; 13:311–315.
8. Piga M, Vacca A, Porru G, Cauli A, Mathieu A. Liver involvement in systemic lupus erythematosus: incidence, clinical course and outcome of lupus hepatitis. Clin Exp Rheumatol. 2010; 28:504–510.

9. Chowdhary VR, Crowson CS, Poterucha JJ, Moder KG. Liver involvement in systemic lupus erythematosus: case review of 40 patients. J Rheumatol. 2008; 35:2159–2164.


10. Park JY, Choi MS, Lim YS, Park JW, Kim SU, Min YW, et al. Clinical features, image findings, and prognosis of inflammatory pseudotumor of the liver: a multicenter experience of 45 cases. Gut Liver. 2014; 8:58–63.


11. Wangkaew S, Lertprasertsuk N, Chotirosniramit A, Louthrenoo W. Hepatic vasculitis presenting with multiple sterile liver abscesses in a patient with systemic lupus erythematosus. Int J Rheum Dis. 2007; 10:64–68.

12. Syed MA, Kim TK, Jang HJ. Portal and hepatic vein thrombosis in liver abscess: CT findings. Eur J Radiol. 2007; 61:513–519.


13. Thietart S, Mekinian A, Delorme S, Lequoy M, Gobert D, Arrivé L, et al. Vasculitis of the hepatic artery: a case of a single-organ vasculitis. Rev Med Interne. 2017; 38:847–849.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download