This article has been
cited by other articles in ScienceCentral.
Dear Editor:
Nivolumab, a programmed death-1 (PD-1) immune checkpoint inhibitor antibody, has demonstrated improved survival over unresectable or metastatic melanoma and locally advanced or metastatic non-small cell lung cancer (NSCLC)
1. This received approval in South Korea on April, 2016, for these cancers. Here, we present a case of lichen planus (LP) after nivolumab treatment in a patient with NSCLC.
A 51-year-old male diagnosed with NSCLC was referred to our dermatology department because of violaceous plaques on face and neck. Pleural invasion had been found although he had undergone chemotherapy (pemetrexed and cisplatin). Accordingly, nivolumab (2 mg/kg/d) had been started and administered every 3 weeks. Three months after the nivolumab treatment, he developed multiple violaceous or dusky brown flat topped plaques on face and neck. The skin lesion did not disappear so that we performed the skin biopsy. The biopsy specimen of his neck demonstrated orthokeratosis, wedge-shaped hypergranulosis, hydropic degeneration of basal layer, and dermal lichenoid lymphocytic infiltration (
Fig. 1). We diagnosed the lesions with LP and started with topical calcineurin inhibitor. A few weeks later, the skin lesions improved markedly. Treatment with nivolumab is currently ongoing. We received the patient's consent form about publishing all photographic materials.
Drug-induced LP is a rare cutaneous side effect of several drugs, such as antimalarials, beta-blockers, gold salts, methyldopa, or quinidine
2. The time from drug administration to the appearance of the lesion varies from one month to one year or more. Typical cutaneous lesions of drug-induced LP are similar to idiopathic LP, with a symmetrical eruption of flat-topped, erythematous or violaceous papules on the trunk and extremities. However, drug-induced LP rarely shows distribution of flexural area, which is common in idiopathic LP. In addition, mucosal involvement is less common in drug-induced LP
3.
Both drug-induced LP and idiopathic LP cannot be distinguished principally by histology. In drug-induced LP, the stratum granulosum is not always hypertrophic, hypergranulosis can be missing and dermal infiltrate may contain eosinophils and plasma cells. However, these differences are often subtle and not reliable
2, as in our case.
LP is a T-cell-mediated chronic inflammatory disease that develops in skin and mucosa
124. Anti-PD-1 therapy induces T-cell activation by inhibiting the suppressive effect of PD-1 signaling on T cells and induces anti-tumor effects in various cancers. Although the pathological mechanisms that induced LP by nivolumab remain unknown, the excess activation of T-cell through nivolumab is a possible explanation
145.
There are several reports of LP associated with Anti-PD-1 therapy (
Table 1)
145. Two patients received pembrolizumab and three patients received nivolumab. LP developed 3 to 12 months after starting Anti-PD-1 therapy. In the present case, skin lesion developed 3 months after nivolumab therapy itself. Two cases were associated with nivolumab after radiotherapy. They have been reported that radiation also affects anti-tumor immunity by induction of cell death chemokine production to recruit T-cell. In all cases, LP improved and almost healed after systemic/topical corticosteroid or topical calcineurin inhibitor. Management of idiopathic LP is challenging, but Anti-PD-1 therapy-induced LP improved by conventional treatment or discontinuation of drug.
In summary, physicians should be aware of the potential development of such cutaneous adverse events when administrating nivolumab therapy.
Figures and Tables
Fig. 1
(A) Violaceous or dusky brown flat-topped plaques arrow on the patient's neck. (B) Orthokeratosis, wedge-shaped hypergranulosis, and saw-toothed irregular elongated rete ridges in the epidermis. Subepidermal cleft and lichenoid lymphocytic infiltration in the dermis (H&E, ×40). (C) Dyskeratotic cells in the epidermis (H&E, ×100).
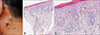
Table 1
Anti-PD-1 therapy-induced lichen planus: review of the literature






 ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download